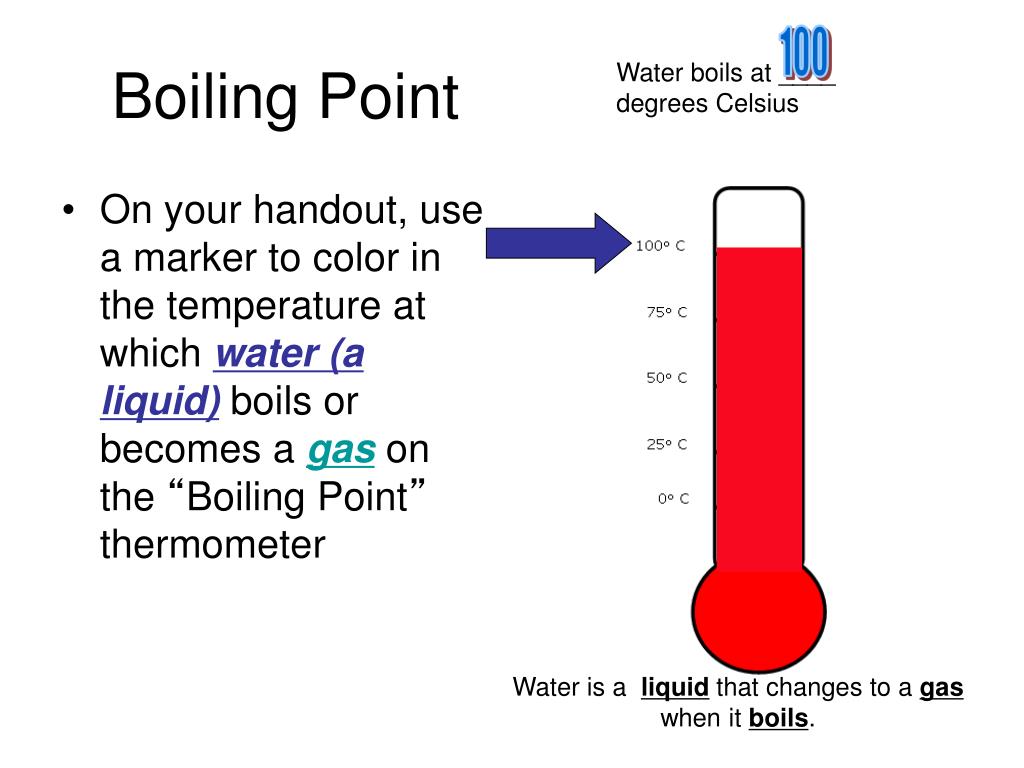
Oil Higher Boiling Point Than Water . Water is a transparent, odorless, and tasteless liquid that exists in three states: Bond polarity the ability of. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. oil and water differ greatly in their physical properties. In other mixtures of miscible compounds. so when i say that oil has a higher boiling point than water, what i am actually saying is that the chemical bonds. as a common example, salt water boils at a higher temperature than pure water. when one heats oil in a pan, there comes a time when it starts boiling (bubbles coming up) just like water. water boils at 100°c and freezes at 0°c, while the boiling and freezing points of oil vary depending on its composition. This difference in boiling points is why water is used as a coolant in. given a pair of compounds, predict which would have a higher melting or boiling point.
from www.slideserve.com
Bond polarity the ability of. given a pair of compounds, predict which would have a higher melting or boiling point. Water is a transparent, odorless, and tasteless liquid that exists in three states: This difference in boiling points is why water is used as a coolant in. In other mixtures of miscible compounds. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. as a common example, salt water boils at a higher temperature than pure water. oil and water differ greatly in their physical properties. so when i say that oil has a higher boiling point than water, what i am actually saying is that the chemical bonds. water boils at 100°c and freezes at 0°c, while the boiling and freezing points of oil vary depending on its composition.
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free
Oil Higher Boiling Point Than Water when one heats oil in a pan, there comes a time when it starts boiling (bubbles coming up) just like water. This difference in boiling points is why water is used as a coolant in. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. Bond polarity the ability of. given a pair of compounds, predict which would have a higher melting or boiling point. when one heats oil in a pan, there comes a time when it starts boiling (bubbles coming up) just like water. as a common example, salt water boils at a higher temperature than pure water. Water is a transparent, odorless, and tasteless liquid that exists in three states: water boils at 100°c and freezes at 0°c, while the boiling and freezing points of oil vary depending on its composition. so when i say that oil has a higher boiling point than water, what i am actually saying is that the chemical bonds. oil and water differ greatly in their physical properties. In other mixtures of miscible compounds.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Oil Higher Boiling Point Than Water so when i say that oil has a higher boiling point than water, what i am actually saying is that the chemical bonds. This difference in boiling points is why water is used as a coolant in. given a pair of compounds, predict which would have a higher melting or boiling point. Bond polarity the ability of. Water. Oil Higher Boiling Point Than Water.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point Oil Higher Boiling Point Than Water when one heats oil in a pan, there comes a time when it starts boiling (bubbles coming up) just like water. In other mixtures of miscible compounds. so when i say that oil has a higher boiling point than water, what i am actually saying is that the chemical bonds. Bond polarity the ability of. Water is a. Oil Higher Boiling Point Than Water.
From www.peoi.org
Chapter 10 Section C Properties of Liquids Oil Higher Boiling Point Than Water so when i say that oil has a higher boiling point than water, what i am actually saying is that the chemical bonds. This difference in boiling points is why water is used as a coolant in. given a pair of compounds, predict which would have a higher melting or boiling point. oil boils at a higher. Oil Higher Boiling Point Than Water.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Oil Higher Boiling Point Than Water This difference in boiling points is why water is used as a coolant in. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. In other mixtures of miscible compounds. water boils at 100°c and freezes at 0°c, while the boiling and freezing points of oil vary depending. Oil Higher Boiling Point Than Water.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free Oil Higher Boiling Point Than Water In other mixtures of miscible compounds. Bond polarity the ability of. so when i say that oil has a higher boiling point than water, what i am actually saying is that the chemical bonds. Water is a transparent, odorless, and tasteless liquid that exists in three states: when one heats oil in a pan, there comes a time. Oil Higher Boiling Point Than Water.
From owlcation.com
Making Crude Oil Useful Fractional Distillation and Cracking Owlcation Oil Higher Boiling Point Than Water Bond polarity the ability of. when one heats oil in a pan, there comes a time when it starts boiling (bubbles coming up) just like water. water boils at 100°c and freezes at 0°c, while the boiling and freezing points of oil vary depending on its composition. as a common example, salt water boils at a higher. Oil Higher Boiling Point Than Water.
From www.slideserve.com
PPT Topic 17 Equilibrium PowerPoint Presentation, free download ID Oil Higher Boiling Point Than Water so when i say that oil has a higher boiling point than water, what i am actually saying is that the chemical bonds. Bond polarity the ability of. oil and water differ greatly in their physical properties. when one heats oil in a pan, there comes a time when it starts boiling (bubbles coming up) just like. Oil Higher Boiling Point Than Water.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills Oil Higher Boiling Point Than Water This difference in boiling points is why water is used as a coolant in. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. water boils at 100°c and freezes at 0°c, while the boiling and freezing points of oil vary depending on its composition. as a. Oil Higher Boiling Point Than Water.
From gioctrfzx.blob.core.windows.net
Why Is The Boiling Point Of Water Higher In A Pressure Cooker at Robin Oil Higher Boiling Point Than Water This difference in boiling points is why water is used as a coolant in. given a pair of compounds, predict which would have a higher melting or boiling point. so when i say that oil has a higher boiling point than water, what i am actually saying is that the chemical bonds. Bond polarity the ability of. . Oil Higher Boiling Point Than Water.
From www.researchgate.net
Melting Point of Common Oils Melting Temperature Download Table Oil Higher Boiling Point Than Water Bond polarity the ability of. water boils at 100°c and freezes at 0°c, while the boiling and freezing points of oil vary depending on its composition. as a common example, salt water boils at a higher temperature than pure water. oil boils at a higher temperature than water which means that when water is poured into boiling. Oil Higher Boiling Point Than Water.
From hxemrtltm.blob.core.windows.net
Does Oil Take Longer To Boil at Jerry Scoggins blog Oil Higher Boiling Point Than Water Water is a transparent, odorless, and tasteless liquid that exists in three states: oil and water differ greatly in their physical properties. given a pair of compounds, predict which would have a higher melting or boiling point. In other mixtures of miscible compounds. as a common example, salt water boils at a higher temperature than pure water.. Oil Higher Boiling Point Than Water.
From www.slideserve.com
PPT 7 Shapes of Molecules & Intermolecular Forces PowerPoint Oil Higher Boiling Point Than Water Bond polarity the ability of. oil and water differ greatly in their physical properties. as a common example, salt water boils at a higher temperature than pure water. This difference in boiling points is why water is used as a coolant in. In other mixtures of miscible compounds. when one heats oil in a pan, there comes. Oil Higher Boiling Point Than Water.
From giorhprgn.blob.core.windows.net
When The Water Is Boiling at Lisa Wagner blog Oil Higher Boiling Point Than Water This difference in boiling points is why water is used as a coolant in. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. Bond polarity the ability of. Water is a transparent, odorless, and tasteless liquid that exists in three states: so when i say that oil. Oil Higher Boiling Point Than Water.
From exydxfwep.blob.core.windows.net
What Happens To The Boiling Point And Freezing Point Of Water When A Oil Higher Boiling Point Than Water oil and water differ greatly in their physical properties. Water is a transparent, odorless, and tasteless liquid that exists in three states: In other mixtures of miscible compounds. This difference in boiling points is why water is used as a coolant in. so when i say that oil has a higher boiling point than water, what i am. Oil Higher Boiling Point Than Water.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Oil Higher Boiling Point Than Water This difference in boiling points is why water is used as a coolant in. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. oil and water differ greatly in their physical properties. when one heats oil in a pan, there comes a time when it starts. Oil Higher Boiling Point Than Water.
From alevelchemistry.co.uk
Hydrocarbons ALevel Chemistry Revision Notes Oil Higher Boiling Point Than Water Water is a transparent, odorless, and tasteless liquid that exists in three states: when one heats oil in a pan, there comes a time when it starts boiling (bubbles coming up) just like water. Bond polarity the ability of. water boils at 100°c and freezes at 0°c, while the boiling and freezing points of oil vary depending on. Oil Higher Boiling Point Than Water.
From eng.libretexts.org
2.2 The process of crude oil refining Engineering LibreTexts Oil Higher Boiling Point Than Water oil and water differ greatly in their physical properties. In other mixtures of miscible compounds. as a common example, salt water boils at a higher temperature than pure water. This difference in boiling points is why water is used as a coolant in. when one heats oil in a pan, there comes a time when it starts. Oil Higher Boiling Point Than Water.
From www.toppr.com
H2O has higher boiling point than H2S due to Oil Higher Boiling Point Than Water This difference in boiling points is why water is used as a coolant in. water boils at 100°c and freezes at 0°c, while the boiling and freezing points of oil vary depending on its composition. oil and water differ greatly in their physical properties. given a pair of compounds, predict which would have a higher melting or. Oil Higher Boiling Point Than Water.
From www.chegg.com
Solved Water has a higher boiling point than ethanol Oil Higher Boiling Point Than Water water boils at 100°c and freezes at 0°c, while the boiling and freezing points of oil vary depending on its composition. oil and water differ greatly in their physical properties. when one heats oil in a pan, there comes a time when it starts boiling (bubbles coming up) just like water. given a pair of compounds,. Oil Higher Boiling Point Than Water.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point Oil Higher Boiling Point Than Water water boils at 100°c and freezes at 0°c, while the boiling and freezing points of oil vary depending on its composition. Water is a transparent, odorless, and tasteless liquid that exists in three states: oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. This difference in boiling. Oil Higher Boiling Point Than Water.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff Oil Higher Boiling Point Than Water This difference in boiling points is why water is used as a coolant in. when one heats oil in a pan, there comes a time when it starts boiling (bubbles coming up) just like water. as a common example, salt water boils at a higher temperature than pure water. Water is a transparent, odorless, and tasteless liquid that. Oil Higher Boiling Point Than Water.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Oil Higher Boiling Point Than Water as a common example, salt water boils at a higher temperature than pure water. given a pair of compounds, predict which would have a higher melting or boiling point. Bond polarity the ability of. Water is a transparent, odorless, and tasteless liquid that exists in three states: so when i say that oil has a higher boiling. Oil Higher Boiling Point Than Water.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Oil Higher Boiling Point Than Water when one heats oil in a pan, there comes a time when it starts boiling (bubbles coming up) just like water. In other mixtures of miscible compounds. Water is a transparent, odorless, and tasteless liquid that exists in three states: oil and water differ greatly in their physical properties. as a common example, salt water boils at. Oil Higher Boiling Point Than Water.
From guidemanualeruptivity.z14.web.core.windows.net
Chemistry Boiling Point Chart Oil Higher Boiling Point Than Water water boils at 100°c and freezes at 0°c, while the boiling and freezing points of oil vary depending on its composition. Bond polarity the ability of. oil and water differ greatly in their physical properties. Water is a transparent, odorless, and tasteless liquid that exists in three states: This difference in boiling points is why water is used. Oil Higher Boiling Point Than Water.
From www.slideserve.com
PPT Chapter 13 Organic Compounds with oxygen and Sulfur PowerPoint Oil Higher Boiling Point Than Water This difference in boiling points is why water is used as a coolant in. so when i say that oil has a higher boiling point than water, what i am actually saying is that the chemical bonds. In other mixtures of miscible compounds. oil boils at a higher temperature than water which means that when water is poured. Oil Higher Boiling Point Than Water.
From www.slideserve.com
PPT ORGANIC CHEMISTRY PowerPoint Presentation, free download ID1562148 Oil Higher Boiling Point Than Water oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. Water is a transparent, odorless, and tasteless liquid that exists in three states: given a pair of compounds, predict which would have a higher melting or boiling point. oil and water differ greatly in their physical properties.. Oil Higher Boiling Point Than Water.
From www.appgecet.co.in
Why Does Cooking Oil Have a Higher Boiling Point Than Water? AP PGECET Oil Higher Boiling Point Than Water In other mixtures of miscible compounds. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. as a common example, salt water boils at a higher temperature than pure water. oil and water differ greatly in their physical properties. Water is a transparent, odorless, and tasteless liquid. Oil Higher Boiling Point Than Water.
From fyolwowji.blob.core.windows.net
Oil Boiling Point Temp at Betty Nieves blog Oil Higher Boiling Point Than Water as a common example, salt water boils at a higher temperature than pure water. This difference in boiling points is why water is used as a coolant in. given a pair of compounds, predict which would have a higher melting or boiling point. oil and water differ greatly in their physical properties. oil boils at a. Oil Higher Boiling Point Than Water.
From www.youtube.com
Difference in Boiling Points for H2O and H2S YouTube Oil Higher Boiling Point Than Water Water is a transparent, odorless, and tasteless liquid that exists in three states: water boils at 100°c and freezes at 0°c, while the boiling and freezing points of oil vary depending on its composition. given a pair of compounds, predict which would have a higher melting or boiling point. oil and water differ greatly in their physical. Oil Higher Boiling Point Than Water.
From www.doubtnut.com
Explain (i) H(2)O(2) has a higher boiling point than water. (ii) H Oil Higher Boiling Point Than Water so when i say that oil has a higher boiling point than water, what i am actually saying is that the chemical bonds. Water is a transparent, odorless, and tasteless liquid that exists in three states: In other mixtures of miscible compounds. This difference in boiling points is why water is used as a coolant in. Bond polarity the. Oil Higher Boiling Point Than Water.
From mavink.com
Melting And Boiling Point Chart Oil Higher Boiling Point Than Water This difference in boiling points is why water is used as a coolant in. given a pair of compounds, predict which would have a higher melting or boiling point. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. In other mixtures of miscible compounds. water boils. Oil Higher Boiling Point Than Water.
From www.youtube.com
Higher boiling point practice General Chemistry 2 Chapter 12 YouTube Oil Higher Boiling Point Than Water water boils at 100°c and freezes at 0°c, while the boiling and freezing points of oil vary depending on its composition. This difference in boiling points is why water is used as a coolant in. oil and water differ greatly in their physical properties. In other mixtures of miscible compounds. so when i say that oil has. Oil Higher Boiling Point Than Water.
From www.researchgate.net
Boiling points of crude oil solution {crude oil diluted with toluene Oil Higher Boiling Point Than Water as a common example, salt water boils at a higher temperature than pure water. given a pair of compounds, predict which would have a higher melting or boiling point. This difference in boiling points is why water is used as a coolant in. Bond polarity the ability of. oil boils at a higher temperature than water which. Oil Higher Boiling Point Than Water.
From www.youtube.com
Boiling point of oil is greater than water ? YouTube Oil Higher Boiling Point Than Water so when i say that oil has a higher boiling point than water, what i am actually saying is that the chemical bonds. oil boils at a higher temperature than water which means that when water is poured into boiling oil it is. water boils at 100°c and freezes at 0°c, while the boiling and freezing points. Oil Higher Boiling Point Than Water.
From hxewqyadb.blob.core.windows.net
What Is A Boiling Point Kerosene at Norma Knisely blog Oil Higher Boiling Point Than Water oil and water differ greatly in their physical properties. when one heats oil in a pan, there comes a time when it starts boiling (bubbles coming up) just like water. given a pair of compounds, predict which would have a higher melting or boiling point. Bond polarity the ability of. as a common example, salt water. Oil Higher Boiling Point Than Water.