Reaction Quotient For Electrochemical Cell . for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and. the nernst equation determines the cell potential of reactions that depend on ph. the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. Sometimes it is helpful to express the nernst equation differently: what is the nernst equation? If h⁺ is involved in the cell reaction, the electrochemical potential.
from www.chegg.com
The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and. If h⁺ is involved in the cell reaction, the electrochemical potential. the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. Sometimes it is helpful to express the nernst equation differently: the nernst equation determines the cell potential of reactions that depend on ph. what is the nernst equation?
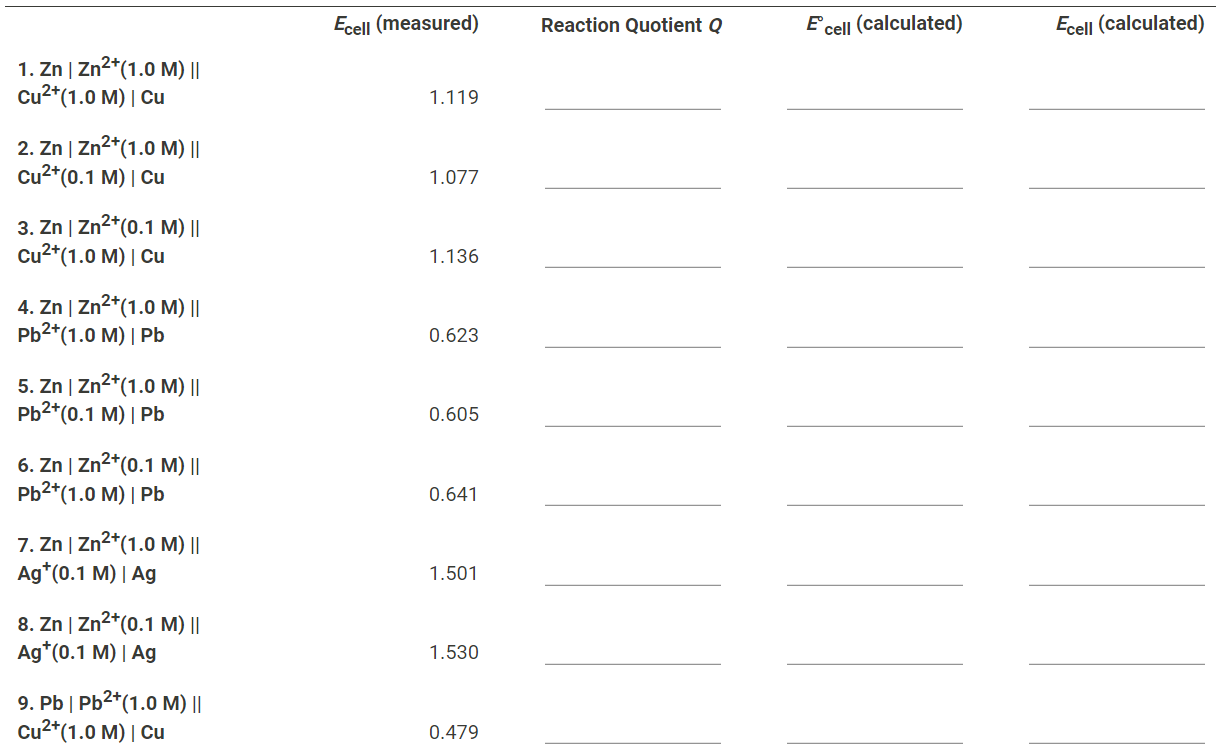
Solved Complete the following table. For each of the 15
Reaction Quotient For Electrochemical Cell q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. what is the nernst equation? q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and. If h⁺ is involved in the cell reaction, the electrochemical potential. Sometimes it is helpful to express the nernst equation differently: the nernst equation determines the cell potential of reactions that depend on ph.
From 2012books.lardbucket.org
Describing Electrochemical Cells Reaction Quotient For Electrochemical Cell If h⁺ is involved in the cell reaction, the electrochemical potential. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and. what is the nernst equation? q = reaction quotient, which is the equilibrium expression with initial concentrations rather. Reaction Quotient For Electrochemical Cell.
From www.youtube.com
Using the Nernst equation Redox reactions and electrochemistry Reaction Quotient For Electrochemical Cell The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. If h⁺ is involved in the cell reaction, the electrochemical potential. Sometimes it is helpful to express the nernst equation differently: q. Reaction Quotient For Electrochemical Cell.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE Reaction Quotient For Electrochemical Cell The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. Sometimes it is helpful to express the nernst equation differently: the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. If h⁺ is involved in the cell reaction, the electrochemical potential. what is. Reaction Quotient For Electrochemical Cell.
From www.slideserve.com
PPT Lecture 13 The Nernst Equation PowerPoint Presentation, free Reaction Quotient For Electrochemical Cell the nernst equation determines the cell potential of reactions that depend on ph. for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. If h⁺ is involved in the. Reaction Quotient For Electrochemical Cell.
From www.numerade.com
SOLVED The charge in coulombs of mole of electrons The electrode in an Reaction Quotient For Electrochemical Cell the nernst equation determines the cell potential of reactions that depend on ph. The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. If h⁺ is involved in the cell reaction, the electrochemical. Reaction Quotient For Electrochemical Cell.
From www.youtube.com
Galvanic cell, Reaction Quotient and the Nernst Equation YouTube Reaction Quotient For Electrochemical Cell for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. the nernst equation determines the cell potential of reactions that depend on ph. The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. the nernst equation provides the relation between the. Reaction Quotient For Electrochemical Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID3975392 Reaction Quotient For Electrochemical Cell what is the nernst equation? q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. Sometimes. Reaction Quotient For Electrochemical Cell.
From salma-has-blevins.blogspot.com
How to Calculate Cell Potential Under Standard Conditions Salmahas Reaction Quotient For Electrochemical Cell q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. the nernst equation determines the cell potential of reactions that depend on ph. If h⁺ is involved in the cell reaction, the electrochemical potential. The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. what is the. Reaction Quotient For Electrochemical Cell.
From general.chemistrysteps.com
Reaction Quotient Q Chemistry Steps Reaction Quotient For Electrochemical Cell q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. the nernst equation determines the cell potential of reactions that depend on ph. Sometimes it is helpful to express the. Reaction Quotient For Electrochemical Cell.
From www.bartleby.com
Answered 6) What standard condition(s) the… bartleby Reaction Quotient For Electrochemical Cell the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. what is the nernst equation? If h⁺ is involved in the cell reaction, the electrochemical potential. Sometimes it is helpful to express the. Reaction Quotient For Electrochemical Cell.
From www.numerade.com
SOLVED Table VC.2 Data and Calculations Reaction quotient, standard Reaction Quotient For Electrochemical Cell the nernst equation determines the cell potential of reactions that depend on ph. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and. the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard. Reaction Quotient For Electrochemical Cell.
From www.nagwa.com
Question Video Selecting the Correct Anode and Cathode Equilibrium Reaction Quotient For Electrochemical Cell what is the nernst equation? the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. Sometimes it is helpful to express the nernst equation differently: The nernst equation calculates electrochemical. Reaction Quotient For Electrochemical Cell.
From www.toppr.com
The value of the reaction quotient, Q, for the cell, Ni(s)Ni^2 + (0 Reaction Quotient For Electrochemical Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and. what is the nernst equation? The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. If h⁺ is involved in the cell reaction, the electrochemical potential.. Reaction Quotient For Electrochemical Cell.
From www.numerade.com
SOLVED What is the value of the reaction quotient, Q, for the voltaic Reaction Quotient For Electrochemical Cell Sometimes it is helpful to express the nernst equation differently: applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and. If h⁺ is involved in the cell reaction, the electrochemical potential. what is the nernst equation? the nernst equation. Reaction Quotient For Electrochemical Cell.
From www.numerade.com
SOLVED HW Ch 20 Pt 3 The Nernst Equation Constants Periodic Table The Reaction Quotient For Electrochemical Cell If h⁺ is involved in the cell reaction, the electrochemical potential. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and. q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. what is. Reaction Quotient For Electrochemical Cell.
From itsreleased.com
Nernst Equation Understanding Electrochemical Equilibrium Reaction Quotient For Electrochemical Cell the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. If h⁺ is involved in the cell reaction, the electrochemical potential. The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. applying the nernst equation to a simple electrochemical cell such as the. Reaction Quotient For Electrochemical Cell.
From www.youtube.com
Daniell_Galvanic_Voltaic Cell 👉 Salt Bridge 👉 Equilibrium constant K Reaction Quotient For Electrochemical Cell for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell. Reaction Quotient For Electrochemical Cell.
From byjus.com
5.How found the reaction quotient (Q) of a cell? Reaction Quotient For Electrochemical Cell q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. what is the nernst equation? the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. Sometimes it is helpful to express the nernst equation differently: The nernst equation calculates electrochemical. Reaction Quotient For Electrochemical Cell.
From saylordotorg.github.io
Electrochemistry Reaction Quotient For Electrochemical Cell for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. Sometimes it is helpful to express the nernst equation differently: what is the nernst equation? applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the. Reaction Quotient For Electrochemical Cell.
From www.slideserve.com
PPT Equilibrium Electrochemistry PowerPoint Presentation, free Reaction Quotient For Electrochemical Cell for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. what is the nernst equation? The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us. Reaction Quotient For Electrochemical Cell.
From www.numerade.com
SOLVED Lab Submission Electrochemistry (36 pts) Voltaic Cells Table Reaction Quotient For Electrochemical Cell If h⁺ is involved in the cell reaction, the electrochemical potential. The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. for cases where we change both [zn 2+] and [cu 2+], we have to refine what we. Reaction Quotient For Electrochemical Cell.
From www.numerade.com
⏩SOLVEDCalculate the reaction quotient, Q, for the cell reaction Reaction Quotient For Electrochemical Cell what is the nernst equation? If h⁺ is involved in the cell reaction, the electrochemical potential. the nernst equation determines the cell potential of reactions that depend on ph. q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. applying the nernst equation to a simple electrochemical cell such as. Reaction Quotient For Electrochemical Cell.
From www.youtube.com
Which of the following are correct for a galvanic cell (Q=Reaction Reaction Quotient For Electrochemical Cell q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how. Reaction Quotient For Electrochemical Cell.
From www.slideserve.com
PPT Chapter 14 Chemical Equilibrium PowerPoint Presentation, free Reaction Quotient For Electrochemical Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and. The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. for cases where we change both [zn 2+] and [cu 2+], we have to refine what. Reaction Quotient For Electrochemical Cell.
From www.numerade.com
SOLVED An electrochemical cell is based on the following two half Reaction Quotient For Electrochemical Cell the nernst equation determines the cell potential of reactions that depend on ph. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and. q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations.. Reaction Quotient For Electrochemical Cell.
From www.youtube.com
Reaction Quotient Q Lecture and Example (Pt. 7) YouTube Reaction Quotient For Electrochemical Cell the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. what is the nernst equation? The nernst equation calculates electrochemical cell potential at any. Reaction Quotient For Electrochemical Cell.
From thechemistrynotes.com
Reaction Quotient (Q) Equation, Calculation, Types, Units Reaction Quotient For Electrochemical Cell the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. the nernst equation determines the cell potential of reactions that depend on ph. q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. applying the nernst equation to a. Reaction Quotient For Electrochemical Cell.
From www.numerade.com
SOLVED [20 Marks] An electrochemical cell containing zinc and copper Reaction Quotient For Electrochemical Cell q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. Sometimes it is helpful to express the nernst equation differently: for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. what is the nernst equation? The nernst equation calculates. Reaction Quotient For Electrochemical Cell.
From www.chegg.com
Solved Complete the following table. For each of the 15 Reaction Quotient For Electrochemical Cell applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and. for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. what is the nernst equation? the. Reaction Quotient For Electrochemical Cell.
From www.numerade.com
Reaction quotient overview Numerade Reaction Quotient For Electrochemical Cell what is the nernst equation? for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. If h⁺ is involved in the cell reaction, the. Reaction Quotient For Electrochemical Cell.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID235007 Reaction Quotient For Electrochemical Cell for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. applying the nernst equation to a simple electrochemical cell such as the zn/cu cell allows us to see how the cell voltage varies as the reaction progresses and. q = reaction quotient, which is the. Reaction Quotient For Electrochemical Cell.
From studylib.net
I. The Reaction Quotient Reaction Quotient For Electrochemical Cell The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. for cases where we change both [zn 2+] and [cu 2+], we have to refine what we mean by moving the system. . Reaction Quotient For Electrochemical Cell.
From www.numerade.com
SOLVED The Nernst equation is one of the most important equations in Reaction Quotient For Electrochemical Cell the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. the nernst equation determines the cell potential of reactions that depend on ph. If h⁺ is involved in the cell reaction, the electrochemical potential. Sometimes it is helpful to express the nernst equation differently: what is. Reaction Quotient For Electrochemical Cell.
From slideplayer.com
Equilibrium Electrochemistry ppt download Reaction Quotient For Electrochemical Cell q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. the nernst equation determines the cell potential of reactions that depend on ph. If h⁺ is involved in the cell reaction, the electrochemical potential. The nernst equation calculates electrochemical cell potential at any known temperature, pressure, and concentration. for cases where. Reaction Quotient For Electrochemical Cell.
From www.numerade.com
SOLVED What is the value of the reaction quotient, Q, for the voltaic Reaction Quotient For Electrochemical Cell q = reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations. the nernst equation provides the relation between the cell potential of an electrochemical cell, the standard cell potential, temperature, and the. Sometimes it is helpful to express the nernst equation differently: applying the nernst equation to a simple electrochemical cell such. Reaction Quotient For Electrochemical Cell.