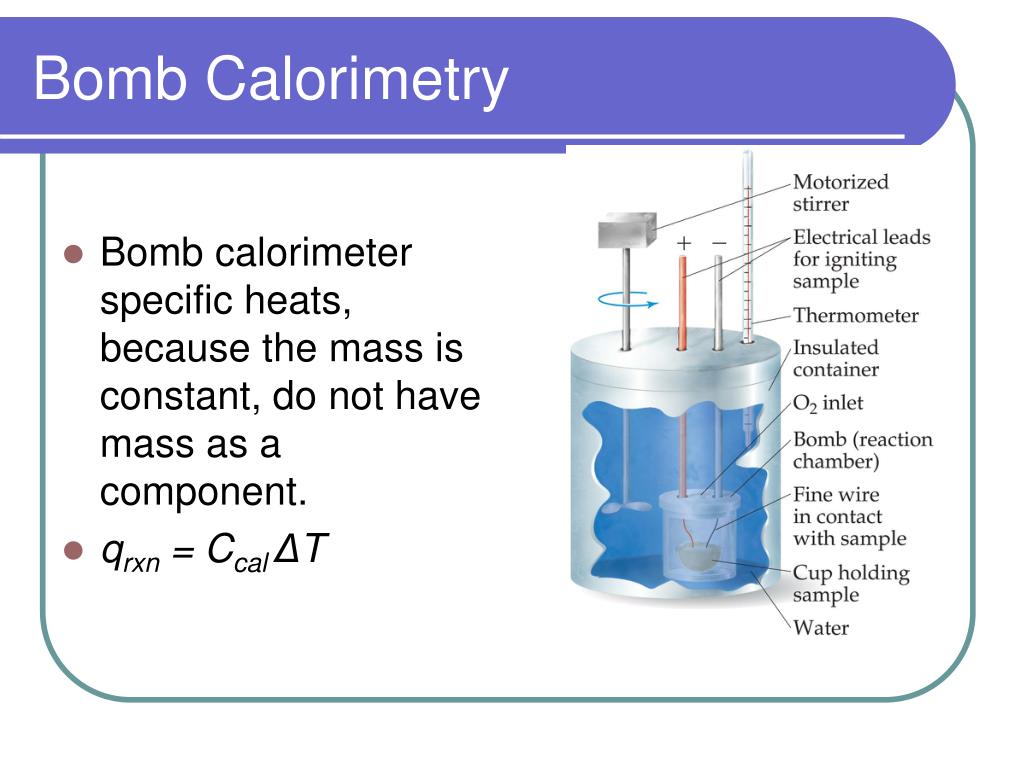
Bomb Calorimeter Reaction . The working of a bomb calorimeter involves a controlled combustion reaction within a sealed container (the bomb) to determine the. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. What is a bomb calorimeter? Chemically, the changes of heat reaction needs to be measured at fixed pressure or volume. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. The bomb calorimeter is referred to as an instrument which is mostly used for the purpose of measuring the heat of reaction that is found having a fixed volume in order to measure the heat which is also termed as the change of internal energy (δe). Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions. A typical bomb calorimetry set up is shown here. The modern bomb calorimeter is a development of the original calorimeter of The reaction takes place in a closed space known as the. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. Now, a bomb calorimeter is an equipment that measures δe for combustion reactions. The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. Bomb calorimetry is a device used for measuring the amount of heat generated from the. This can be seen in the equation of.
from www.slideserve.com
The reaction takes place in a closed space known as the. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. The bomb calorimeter is referred to as an instrument which is mostly used for the purpose of measuring the heat of reaction that is found having a fixed volume in order to measure the heat which is also termed as the change of internal energy (δe). The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Chemically, the changes of heat reaction needs to be measured at fixed pressure or volume. A typical bomb calorimetry set up is shown here. Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions. The working of a bomb calorimeter involves a controlled combustion reaction within a sealed container (the bomb) to determine the. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter.
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation
Bomb Calorimeter Reaction Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Bomb calorimetry is a device used for measuring the amount of heat generated from the. Chemically, the changes of heat reaction needs to be measured at fixed pressure or volume. This can be seen in the equation of. Now, a bomb calorimeter is an equipment that measures δe for combustion reactions. The working of a bomb calorimeter involves a controlled combustion reaction within a sealed container (the bomb) to determine the. The reaction takes place in a closed space known as the. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. The bomb calorimeter is referred to as an instrument which is mostly used for the purpose of measuring the heat of reaction that is found having a fixed volume in order to measure the heat which is also termed as the change of internal energy (δe). The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions. A typical bomb calorimetry set up is shown here. What is a bomb calorimeter? The modern bomb calorimeter is a development of the original calorimeter of The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube Bomb Calorimeter Reaction Bomb calorimetry is a device used for measuring the amount of heat generated from the. Now, a bomb calorimeter is an equipment that measures δe for combustion reactions. A typical bomb calorimetry set up is shown here. Chemically, the changes of heat reaction needs to be measured at fixed pressure or volume. The reaction is contained in a heavy metallic. Bomb Calorimeter Reaction.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation, free download ID3206969 Bomb Calorimeter Reaction Bomb calorimetry is a device used for measuring the amount of heat generated from the. The bomb calorimeter is referred to as an instrument which is mostly used for the purpose of measuring the heat of reaction that is found having a fixed volume in order to measure the heat which is also termed as the change of internal energy. Bomb Calorimeter Reaction.
From socratic.org
What is the change in internal energy of reaction when "0.721 g" of Bomb Calorimeter Reaction This can be seen in the equation of. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions. A typical bomb calorimetry set up is shown here. What is a bomb calorimeter? The working of a. Bomb Calorimeter Reaction.
From www.chegg.com
Solved Combustion (bomb) calorimeter. In an experiment, a Bomb Calorimeter Reaction The reaction takes place in a closed space known as the. The bomb calorimeter is referred to as an instrument which is mostly used for the purpose of measuring the heat of reaction that is found having a fixed volume in order to measure the heat which is also termed as the change of internal energy (δe). Bomb calorimeter an. Bomb Calorimeter Reaction.
From tukioka-clinic.com
😂 Soda can calorimeter sources of error. What Is a Calorimeter & What Bomb Calorimeter Reaction The modern bomb calorimeter is a development of the original calorimeter of Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions. The working of a bomb calorimeter involves a controlled combustion reaction within a sealed container (the bomb) to determine the. The reaction is contained in. Bomb Calorimeter Reaction.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Bomb Calorimeter Reaction What is a bomb calorimeter? Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. Now, a bomb calorimeter is an equipment that measures δe for combustion reactions. The bomb calorimeter is referred to as an instrument which is. Bomb Calorimeter Reaction.
From people.chem.umass.edu
to Adobe GoLive 6 Bomb Calorimeter Reaction This can be seen in the equation of. The modern bomb calorimeter is a development of the original calorimeter of Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction. Bomb Calorimeter Reaction.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Bomb Calorimeter Reaction This can be seen in the equation of. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. The modern bomb calorimeter is a development of the original calorimeter of A typical bomb calorimetry set up is shown here.. Bomb Calorimeter Reaction.
From www.slideserve.com
PPT TOPIC 8 THERMOCHEMISTRY PowerPoint Presentation, free download Bomb Calorimeter Reaction A typical bomb calorimetry set up is shown here. Chemically, the changes of heat reaction needs to be measured at fixed pressure or volume. The reaction takes place in a closed space known as the. Now, a bomb calorimeter is an equipment that measures δe for combustion reactions. This can be seen in the equation of. The modern bomb calorimeter. Bomb Calorimeter Reaction.
From www.chegg.com
Solved The Model Bomb Calorimetry Motorized stirrer Bomb Calorimeter Reaction Chemically, the changes of heat reaction needs to be measured at fixed pressure or volume. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Now, a bomb calorimeter is an equipment that measures δe for combustion reactions. The reaction takes place in a closed space known as the. Bomb calorimeter an. Bomb Calorimeter Reaction.
From www.pinterest.com
ConstantVolume Calorimetry for more precise work than the coffeecup Bomb Calorimeter Reaction Now, a bomb calorimeter is an equipment that measures δe for combustion reactions. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Chemically, the changes of heat reaction needs to be measured at fixed pressure or volume. The bomb calorimeter is referred to as an instrument which is mostly used for. Bomb Calorimeter Reaction.
From www.youtube.com
For the reaction of one mole of zinc dust with one mole of sulphuric Bomb Calorimeter Reaction The reaction takes place in a closed space known as the. The modern bomb calorimeter is a development of the original calorimeter of The bomb calorimeter is referred to as an instrument which is mostly used for the purpose of measuring the heat of reaction that is found having a fixed volume in order to measure the heat which is. Bomb Calorimeter Reaction.
From courses.lumenlearning.com
Calorimetry Chemistry Bomb Calorimeter Reaction This can be seen in the equation of. Chemically, the changes of heat reaction needs to be measured at fixed pressure or volume. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions. A typical bomb. Bomb Calorimeter Reaction.
From www.animalia-life.club
Calorimeter Diagram Bomb Calorimeter Reaction A typical bomb calorimetry set up is shown here. The modern bomb calorimeter is a development of the original calorimeter of Chemically, the changes of heat reaction needs to be measured at fixed pressure or volume. What is a bomb calorimeter? Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a. Bomb Calorimeter Reaction.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Bomb Calorimeter Reaction The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. A typical bomb calorimetry set up is shown here. Now, a bomb calorimeter is an equipment that measures δe for combustion reactions. The reaction takes place in a closed space known as the. The modern bomb calorimeter is a development of. Bomb Calorimeter Reaction.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimeter Reaction A typical bomb calorimetry set up is shown here. Chemically, the changes of heat reaction needs to be measured at fixed pressure or volume. Bomb calorimetry is a device used for measuring the amount of heat generated from the. The working of a bomb calorimeter involves a controlled combustion reaction within a sealed container (the bomb) to determine the. The. Bomb Calorimeter Reaction.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter Reaction What is a bomb calorimeter? Bomb calorimeter an apparatus primarily used for measuring heats of combustion. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. This can be seen in the equation of. The bomb calorimeter is referred to as an instrument which is mostly used for the purpose of measuring. Bomb Calorimeter Reaction.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Bomb Calorimeter Reaction This can be seen in the equation of. Bomb calorimetry is a device used for measuring the amount of heat generated from the. The modern bomb calorimeter is a development of the original calorimeter of What is a bomb calorimeter? Bomb calorimeter an apparatus primarily used for measuring heats of combustion. The working of a bomb calorimeter involves a controlled. Bomb Calorimeter Reaction.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Bomb Calorimeter Reaction The working of a bomb calorimeter involves a controlled combustion reaction within a sealed container (the bomb) to determine the. Bomb calorimetry is a device used for measuring the amount of heat generated from the. The reaction takes place in a closed space known as the. This can be seen in the equation of. The modern bomb calorimeter is a. Bomb Calorimeter Reaction.
From courses.lumenlearning.com
Calorimetry Chemistry I Bomb Calorimeter Reaction Now, a bomb calorimeter is an equipment that measures δe for combustion reactions. Bomb calorimetry is a device used for measuring the amount of heat generated from the. The modern bomb calorimeter is a development of the original calorimeter of The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. A typical. Bomb Calorimeter Reaction.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimeter Reaction This can be seen in the equation of. The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. The working of a bomb calorimeter involves a controlled combustion reaction within a sealed container (the bomb) to determine the. What is a bomb calorimeter? Bomb calorimetry is a device used for measuring. Bomb Calorimeter Reaction.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation Bomb Calorimeter Reaction The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. The reaction takes place in a closed space known as the. The working of a bomb calorimeter involves a controlled combustion reaction within a sealed container (the bomb) to determine the. This can be seen in the equation of. The bomb. Bomb Calorimeter Reaction.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Bomb Calorimeter Reaction What is a bomb calorimeter? The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. The bomb calorimeter is referred to as an instrument which is mostly used for the purpose of measuring the heat of reaction that is found having a fixed volume in order to measure the heat which. Bomb Calorimeter Reaction.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Bomb Calorimeter Reaction What is a bomb calorimeter? Chemically, the changes of heat reaction needs to be measured at fixed pressure or volume. The working of a bomb calorimeter involves a controlled combustion reaction within a sealed container (the bomb) to determine the. The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. The. Bomb Calorimeter Reaction.
From www.shutterstock.com
Bomb Calorimeter Vector Illustration Labeled Educational Stock Vector Bomb Calorimeter Reaction Bomb calorimetry is a device used for measuring the amount of heat generated from the. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. The bomb calorimeter is referred to as an. Bomb Calorimeter Reaction.
From chemistry.about.com
Coffee Cup and Bomb Calorimetry Bomb Calorimeter Reaction Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. The bomb calorimeter is referred to as an instrument which is mostly used for the purpose of measuring the heat of reaction that is found having a fixed volume in order to measure the heat which is also termed as. Bomb Calorimeter Reaction.
From drivenheisenberg.blogspot.com
Which Diagram Is A Bomb Calorimeter Drivenheisenberg Bomb Calorimeter Reaction The working of a bomb calorimeter involves a controlled combustion reaction within a sealed container (the bomb) to determine the. The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. The reaction takes place in a closed space known as the. Bomb calorimetry is used predominantly to measure the heat evolved. Bomb Calorimeter Reaction.
From www.studypool.com
SOLUTION Bomb calorimeter study material Studypool Bomb Calorimeter Reaction The reaction takes place in a closed space known as the. Now, a bomb calorimeter is an equipment that measures δe for combustion reactions. What is a bomb calorimeter? Bomb calorimeter an apparatus primarily used for measuring heats of combustion. The modern bomb calorimeter is a development of the original calorimeter of The bomb calorimeter is referred to as an. Bomb Calorimeter Reaction.
From saylordotorg.github.io
Calorimetry Bomb Calorimeter Reaction The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions. This can be seen in the equation of. The reaction is contained in a heavy metallic container (the bomb). Bomb Calorimeter Reaction.
From www.slideserve.com
PPT Reaction carried out under constant volume. Use a bomb Bomb Calorimeter Reaction The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. What is a bomb calorimeter? Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be. Bomb Calorimeter Reaction.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Bomb Calorimeter Reaction Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Bomb calorimetry is a device used for measuring the amount of heat generated from the. A typical bomb calorimetry set up is shown here. Chemically, the changes of heat reaction needs to be measured at fixed pressure or volume. Bomb calorimetry is used predominantly to measure the heat evolved. Bomb Calorimeter Reaction.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimeter Reaction The modern bomb calorimeter is a development of the original calorimeter of Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions. Now, a bomb calorimeter is an equipment that measures δe for combustion reactions. This can be seen in the equation of. What is a bomb. Bomb Calorimeter Reaction.
From www.youtube.com
Bomb Calorimetry YouTube Bomb Calorimeter Reaction Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Chemically, the changes of heat reaction needs to be measured at fixed pressure or volume. The bomb calorimeter is referred to as an instrument which is mostly used for the purpose of measuring the heat of reaction that is found having a fixed volume in order to measure the. Bomb Calorimeter Reaction.
From shaunmwilliams.com
Chapter 6 Presentation Bomb Calorimeter Reaction Chemically, the changes of heat reaction needs to be measured at fixed pressure or volume. What is a bomb calorimeter? The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. The bomb calorimeter is referred to as an instrument which. Bomb Calorimeter Reaction.
From www.chegg.com
Solved Question 12 0 / 1 pts In a bomb calorimeter Bomb Calorimeter Reaction The bomb calorimeter is referred to as an instrument which is mostly used for the purpose of measuring the heat of reaction that is found having a fixed volume in order to measure the heat which is also termed as the change of internal energy (δe). A typical bomb calorimetry set up is shown here. The reaction takes place in. Bomb Calorimeter Reaction.