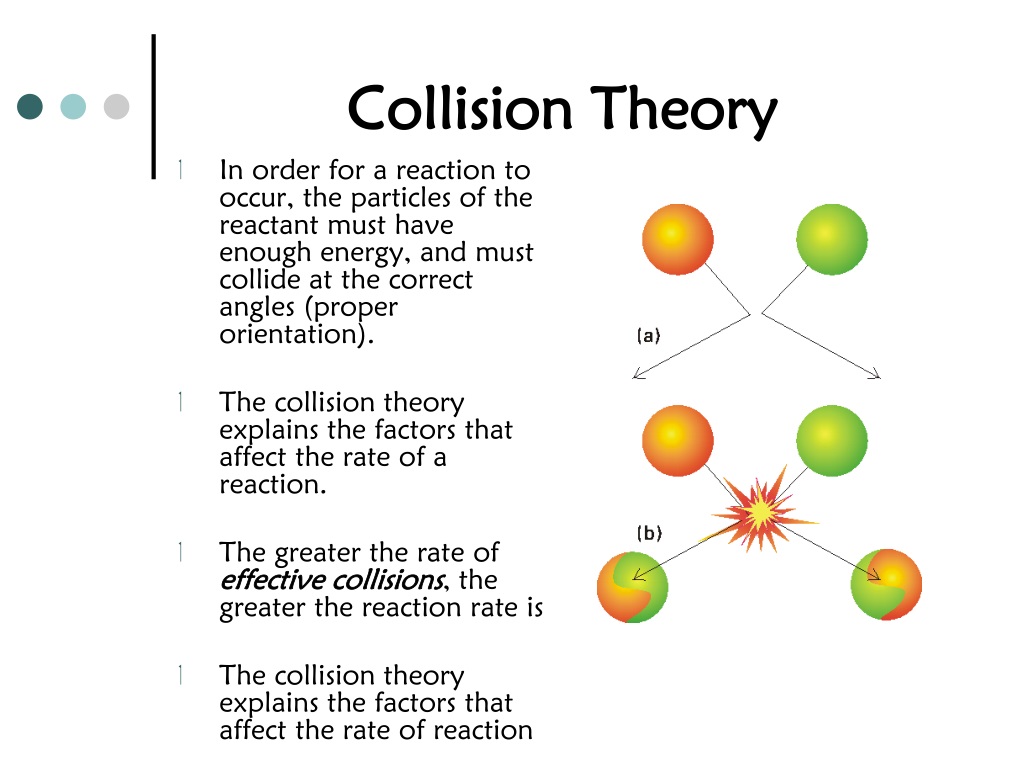
Collision And Effect . We have defined momentum to be the product of mass and velocity. By the end of this section, you will be able to: As in any interaction, a collision results in a force being applied to the two colliding. Describe what an impulse does. Collision theory explains why most reaction rates increase as concentrations increase. When discussing conservation of momentum, we considered examples in which two objects collide and stick. Consider a first particle with mass \ (\mathrm {m_1}\) and velocity \ (\mathrm {v_ {1i}}\) and a second particle with mass \ (\mathrm {m_2}\) and velocity \ (\mathrm {v_ {2i}}\). Therefore, if an object’s velocity should change (due to the application of a force on the object), then necessarily, its momentum changes as well. Correctly label a collision as elastic or inelastic. The mathematics of an elastic collision is best demonstrated through an example. A collision is an interaction between two objects that have made contact (usually) with each other. Use kinetic energy along with. Identify the type of collision. Describe what an impulse does. Explain what an impulse is, physically.
from www.slideserve.com
Explain what an impulse is, physically. Describe what an impulse does. Identify the type of collision. When discussing conservation of momentum, we considered examples in which two objects collide and stick. Describe what an impulse does. Collision theory explains why most reaction rates increase as concentrations increase. A collision is an interaction between two objects that have made contact (usually) with each other. Use kinetic energy along with. With an increase in the concentration of any reacting. As in any interaction, a collision results in a force being applied to the two colliding.
PPT Collision Theory PowerPoint Presentation, free download ID9507407
Collision And Effect When discussing conservation of momentum, we considered examples in which two objects collide and stick. Explain what an impulse is, physically. Describe what an impulse does. A collision is an interaction between two objects that have made contact (usually) with each other. Use kinetic energy along with. We have defined momentum to be the product of mass and velocity. By the end of this section, you will be able to: Identify the type of collision. The mathematics of an elastic collision is best demonstrated through an example. As in any interaction, a collision results in a force being applied to the two colliding. Collision theory explains why most reaction rates increase as concentrations increase. Correctly label a collision as elastic or inelastic. Describe what an impulse does. Consider a first particle with mass \ (\mathrm {m_1}\) and velocity \ (\mathrm {v_ {1i}}\) and a second particle with mass \ (\mathrm {m_2}\) and velocity \ (\mathrm {v_ {2i}}\). Therefore, if an object’s velocity should change (due to the application of a force on the object), then necessarily, its momentum changes as well. When discussing conservation of momentum, we considered examples in which two objects collide and stick.
From www.slideserve.com
PPT Collision Theory PowerPoint Presentation, free download ID9507407 Collision And Effect When discussing conservation of momentum, we considered examples in which two objects collide and stick. By the end of this section, you will be able to: Correctly label a collision as elastic or inelastic. Identify the type of collision. Consider a first particle with mass \ (\mathrm {m_1}\) and velocity \ (\mathrm {v_ {1i}}\) and a second particle with mass. Collision And Effect.
From www.slideserve.com
PPT Chemical Reactions and Collision Theory PowerPoint Presentation Collision And Effect We have defined momentum to be the product of mass and velocity. With an increase in the concentration of any reacting. Therefore, if an object’s velocity should change (due to the application of a force on the object), then necessarily, its momentum changes as well. Correctly label a collision as elastic or inelastic. By the end of this section, you. Collision And Effect.
From pngtree.com
Beam Collision Cool Light Effect, Light Beam, Collision, Light Effect Collision And Effect Consider a first particle with mass \ (\mathrm {m_1}\) and velocity \ (\mathrm {v_ {1i}}\) and a second particle with mass \ (\mathrm {m_2}\) and velocity \ (\mathrm {v_ {2i}}\). Describe what an impulse does. By the end of this section, you will be able to: Identify the type of collision. We have defined momentum to be the product of. Collision And Effect.
From integrative-innovation.net
Innovation and Serendipity Integrative Innovation Collision And Effect Identify the type of collision. As in any interaction, a collision results in a force being applied to the two colliding. Describe what an impulse does. A collision is an interaction between two objects that have made contact (usually) with each other. Use kinetic energy along with. Collision theory explains why most reaction rates increase as concentrations increase. When discussing. Collision And Effect.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Collision And Effect Use kinetic energy along with. Explain what an impulse is, physically. When discussing conservation of momentum, we considered examples in which two objects collide and stick. Describe what an impulse does. Identify the type of collision. With an increase in the concentration of any reacting. A collision is an interaction between two objects that have made contact (usually) with each. Collision And Effect.
From www.gamedevmarket.net
Collision effect V2 GameDev Market Collision And Effect Therefore, if an object’s velocity should change (due to the application of a force on the object), then necessarily, its momentum changes as well. Describe what an impulse does. Identify the type of collision. We have defined momentum to be the product of mass and velocity. A collision is an interaction between two objects that have made contact (usually) with. Collision And Effect.
From quizlet.com
Contrast types of collisions. Quizlet Collision And Effect With an increase in the concentration of any reacting. Therefore, if an object’s velocity should change (due to the application of a force on the object), then necessarily, its momentum changes as well. We have defined momentum to be the product of mass and velocity. By the end of this section, you will be able to: When discussing conservation of. Collision And Effect.
From quizizz.com
Collision Theory and Reaction Rate Science Quizizz Collision And Effect Use kinetic energy along with. Describe what an impulse does. Explain what an impulse is, physically. Therefore, if an object’s velocity should change (due to the application of a force on the object), then necessarily, its momentum changes as well. Describe what an impulse does. Collision theory explains why most reaction rates increase as concentrations increase. By the end of. Collision And Effect.
From www.slideserve.com
PPT Chemical Reactions and Collision Theory PowerPoint Presentation Collision And Effect When discussing conservation of momentum, we considered examples in which two objects collide and stick. A collision is an interaction between two objects that have made contact (usually) with each other. Explain what an impulse is, physically. Use kinetic energy along with. Describe what an impulse does. Consider a first particle with mass \ (\mathrm {m_1}\) and velocity \ (\mathrm. Collision And Effect.
From www.freepik.com
Premium Vector Lightning collision vector Collision And Effect Correctly label a collision as elastic or inelastic. The mathematics of an elastic collision is best demonstrated through an example. Describe what an impulse does. Therefore, if an object’s velocity should change (due to the application of a force on the object), then necessarily, its momentum changes as well. Collision theory explains why most reaction rates increase as concentrations increase.. Collision And Effect.
From giphy.com
Collision GIF Find & Share on GIPHY Collision And Effect When discussing conservation of momentum, we considered examples in which two objects collide and stick. With an increase in the concentration of any reacting. Describe what an impulse does. Describe what an impulse does. Collision theory explains why most reaction rates increase as concentrations increase. Correctly label a collision as elastic or inelastic. As in any interaction, a collision results. Collision And Effect.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.9.1 Collision Theory & Rates翰林国际教育 Collision And Effect Therefore, if an object’s velocity should change (due to the application of a force on the object), then necessarily, its momentum changes as well. The mathematics of an elastic collision is best demonstrated through an example. Consider a first particle with mass \ (\mathrm {m_1}\) and velocity \ (\mathrm {v_ {1i}}\) and a second particle with mass \ (\mathrm {m_2}\). Collision And Effect.
From mmerevise.co.uk
Collision Theory and Reaction Rates MME Collision And Effect A collision is an interaction between two objects that have made contact (usually) with each other. The mathematics of an elastic collision is best demonstrated through an example. Use kinetic energy along with. Describe what an impulse does. Describe what an impulse does. Correctly label a collision as elastic or inelastic. Consider a first particle with mass \ (\mathrm {m_1}\). Collision And Effect.
From saylordotorg.github.io
The Collision Model of Chemical Collision And Effect With an increase in the concentration of any reacting. Explain what an impulse is, physically. A collision is an interaction between two objects that have made contact (usually) with each other. Consider a first particle with mass \ (\mathrm {m_1}\) and velocity \ (\mathrm {v_ {1i}}\) and a second particle with mass \ (\mathrm {m_2}\) and velocity \ (\mathrm {v_. Collision And Effect.
From www.youtube.com
Factors Affecting Rate of Reaction + Collision Theory GCSE Science Collision And Effect Describe what an impulse does. We have defined momentum to be the product of mass and velocity. Collision theory explains why most reaction rates increase as concentrations increase. When discussing conservation of momentum, we considered examples in which two objects collide and stick. A collision is an interaction between two objects that have made contact (usually) with each other. Correctly. Collision And Effect.
From slideplayer.com
Collision and Explosion ppt download Collision And Effect The mathematics of an elastic collision is best demonstrated through an example. By the end of this section, you will be able to: Identify the type of collision. When discussing conservation of momentum, we considered examples in which two objects collide and stick. Collision theory explains why most reaction rates increase as concentrations increase. Describe what an impulse does. Correctly. Collision And Effect.
From www.ck12.org
Collision Theory CK12 Foundation Collision And Effect Use kinetic energy along with. As in any interaction, a collision results in a force being applied to the two colliding. Describe what an impulse does. Consider a first particle with mass \ (\mathrm {m_1}\) and velocity \ (\mathrm {v_ {1i}}\) and a second particle with mass \ (\mathrm {m_2}\) and velocity \ (\mathrm {v_ {2i}}\). Correctly label a collision. Collision And Effect.
From www.sciencefacts.net
Inelastic Collision Definition, Formula, and Examples Collision And Effect Describe what an impulse does. A collision is an interaction between two objects that have made contact (usually) with each other. By the end of this section, you will be able to: We have defined momentum to be the product of mass and velocity. Correctly label a collision as elastic or inelastic. Explain what an impulse is, physically. With an. Collision And Effect.
From www.researchgate.net
Exaggerated collision effect between two particles Download Collision And Effect Explain what an impulse is, physically. Correctly label a collision as elastic or inelastic. Identify the type of collision. Consider a first particle with mass \ (\mathrm {m_1}\) and velocity \ (\mathrm {v_ {1i}}\) and a second particle with mass \ (\mathrm {m_2}\) and velocity \ (\mathrm {v_ {2i}}\). As in any interaction, a collision results in a force being. Collision And Effect.
From cartoondealer.com
Collision And Life Pictured As A Word Collision And A Wreck Ball To Collision And Effect As in any interaction, a collision results in a force being applied to the two colliding. Therefore, if an object’s velocity should change (due to the application of a force on the object), then necessarily, its momentum changes as well. Collision theory explains why most reaction rates increase as concentrations increase. By the end of this section, you will be. Collision And Effect.
From physics.educour.in
Elastic and Inelastic Collision Class 11 CBSE CBSE Physics Collision And Effect Collision theory explains why most reaction rates increase as concentrations increase. We have defined momentum to be the product of mass and velocity. Explain what an impulse is, physically. The mathematics of an elastic collision is best demonstrated through an example. Use kinetic energy along with. As in any interaction, a collision results in a force being applied to the. Collision And Effect.
From www.youtube.com
COLLISION Physics Animation YouTube Collision And Effect Identify the type of collision. Correctly label a collision as elastic or inelastic. We have defined momentum to be the product of mass and velocity. The mathematics of an elastic collision is best demonstrated through an example. A collision is an interaction between two objects that have made contact (usually) with each other. With an increase in the concentration of. Collision And Effect.
From www.britannica.com
Compton effect Definition, Formula, & Facts Britannica Collision And Effect Therefore, if an object’s velocity should change (due to the application of a force on the object), then necessarily, its momentum changes as well. Collision theory explains why most reaction rates increase as concentrations increase. The mathematics of an elastic collision is best demonstrated through an example. Describe what an impulse does. Use kinetic energy along with. Consider a first. Collision And Effect.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Collision And Effect Consider a first particle with mass \ (\mathrm {m_1}\) and velocity \ (\mathrm {v_ {1i}}\) and a second particle with mass \ (\mathrm {m_2}\) and velocity \ (\mathrm {v_ {2i}}\). Correctly label a collision as elastic or inelastic. Use kinetic energy along with. The mathematics of an elastic collision is best demonstrated through an example. With an increase in the. Collision And Effect.
From www.youtube.com
6.2.3 Describe the collision theory. YouTube Collision And Effect Identify the type of collision. Consider a first particle with mass \ (\mathrm {m_1}\) and velocity \ (\mathrm {v_ {1i}}\) and a second particle with mass \ (\mathrm {m_2}\) and velocity \ (\mathrm {v_ {2i}}\). Describe what an impulse does. Collision theory explains why most reaction rates increase as concentrations increase. Therefore, if an object’s velocity should change (due to. Collision And Effect.
From www.diffzy.com
Inelastic vs. Elastic Collisions What's The Difference (With Table) Collision And Effect Correctly label a collision as elastic or inelastic. Describe what an impulse does. The mathematics of an elastic collision is best demonstrated through an example. Consider a first particle with mass \ (\mathrm {m_1}\) and velocity \ (\mathrm {v_ {1i}}\) and a second particle with mass \ (\mathrm {m_2}\) and velocity \ (\mathrm {v_ {2i}}\). With an increase in the. Collision And Effect.
From www.slideserve.com
PPT PowerPoint Presentation, free download ID1991748 Collision And Effect With an increase in the concentration of any reacting. Collision theory explains why most reaction rates increase as concentrations increase. By the end of this section, you will be able to: Identify the type of collision. A collision is an interaction between two objects that have made contact (usually) with each other. Therefore, if an object’s velocity should change (due. Collision And Effect.
From www.freepik.com
Free Vector Realistic two lights collision effect Collision And Effect We have defined momentum to be the product of mass and velocity. Collision theory explains why most reaction rates increase as concentrations increase. By the end of this section, you will be able to: Describe what an impulse does. With an increase in the concentration of any reacting. Describe what an impulse does. As in any interaction, a collision results. Collision And Effect.
From cartoondealer.com
Collision And Life Pictured As A Word Collision And A Wreck Ball To Collision And Effect The mathematics of an elastic collision is best demonstrated through an example. By the end of this section, you will be able to: Identify the type of collision. A collision is an interaction between two objects that have made contact (usually) with each other. We have defined momentum to be the product of mass and velocity. Collision theory explains why. Collision And Effect.
From mmerevise.co.uk
Collision Theory and Reaction Rates MME Collision And Effect Describe what an impulse does. When discussing conservation of momentum, we considered examples in which two objects collide and stick. The mathematics of an elastic collision is best demonstrated through an example. A collision is an interaction between two objects that have made contact (usually) with each other. Identify the type of collision. Collision theory explains why most reaction rates. Collision And Effect.
From www.pinterest.com
Simple Collision Theory Collision theory, Theories, Energy activities Collision And Effect As in any interaction, a collision results in a force being applied to the two colliding. A collision is an interaction between two objects that have made contact (usually) with each other. When discussing conservation of momentum, we considered examples in which two objects collide and stick. Describe what an impulse does. Consider a first particle with mass \ (\mathrm. Collision And Effect.
From blogs.glowscotland.org.uk
Collision Theory and Rate of Reaction Higher Chemistry Unit 1 Collision And Effect With an increase in the concentration of any reacting. We have defined momentum to be the product of mass and velocity. Identify the type of collision. Collision theory explains why most reaction rates increase as concentrations increase. By the end of this section, you will be able to: Use kinetic energy along with. Consider a first particle with mass \. Collision And Effect.
From www.slideserve.com
PPT Momentum and Collisions PowerPoint Presentation, free download Collision And Effect Use kinetic energy along with. With an increase in the concentration of any reacting. The mathematics of an elastic collision is best demonstrated through an example. Describe what an impulse does. A collision is an interaction between two objects that have made contact (usually) with each other. Collision theory explains why most reaction rates increase as concentrations increase. By the. Collision And Effect.
From courses.lumenlearning.com
Collision Theory Chemistry Collision And Effect Correctly label a collision as elastic or inelastic. The mathematics of an elastic collision is best demonstrated through an example. With an increase in the concentration of any reacting. A collision is an interaction between two objects that have made contact (usually) with each other. Describe what an impulse does. As in any interaction, a collision results in a force. Collision And Effect.
From www.researchgate.net
Collision geometry showing the parameters used to describe both highly Collision And Effect Describe what an impulse does. By the end of this section, you will be able to: Correctly label a collision as elastic or inelastic. Use kinetic energy along with. A collision is an interaction between two objects that have made contact (usually) with each other. Identify the type of collision. Explain what an impulse is, physically. Describe what an impulse. Collision And Effect.