Electrode Battery Basics . Learn more about its design in this. Disposable, or primary, batteries, in which the electrode reactions are effectively. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. there are two basic kinds of batteries: In many battery systems, including lead. a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. The battery uses two dissimilar metals (electrodes) and an electrolyte to create a. electrodes and electrolyte: batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. the role of electrodes in the transfer of energy.
from inside.lgensol.com
In many battery systems, including lead. electrodes and electrolyte: Disposable, or primary, batteries, in which the electrode reactions are effectively. Learn more about its design in this. The battery uses two dissimilar metals (electrodes) and an electrolyte to create a. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. there are two basic kinds of batteries: a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. the role of electrodes in the transfer of energy. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical.
How to Make a Battery Step1. Electrode Manufacturing Battery LAB
Electrode Battery Basics electrodes and electrolyte: Learn more about its design in this. there are two basic kinds of batteries: Disposable, or primary, batteries, in which the electrode reactions are effectively. the role of electrodes in the transfer of energy. electrodes and electrolyte: The battery uses two dissimilar metals (electrodes) and an electrolyte to create a. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. In many battery systems, including lead. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. a battery, which is an electric cell, is a device that produces electricity from a chemical reaction.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrode Battery Basics electrodes and electrolyte: batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. The battery uses two dissimilar metals (electrodes) and an electrolyte to create a. there are two basic kinds of batteries: one electrode, known as the cathode, connects to the positive end of the battery and is. Electrode Battery Basics.
From usermanualtractors.z1.web.core.windows.net
What Happens At The Cathode In Electrolysis Electrode Battery Basics batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. Learn more about its design in this. the role of electrodes in the transfer of energy. The battery uses two dissimilar metals (electrodes) and an electrolyte to create a. one electrode, known as the cathode, connects to the positive end. Electrode Battery Basics.
From wiredataoverheadoa.z22.web.core.windows.net
Liion Battery Pack Circuit Diagram Electrode Battery Basics the role of electrodes in the transfer of energy. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. Learn more about its design in. Electrode Battery Basics.
From www.thoughtco.com
How to Define Anode and Cathode Electrode Battery Basics a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. electrodes and electrolyte: the role of electrodes in the transfer of energy. there. Electrode Battery Basics.
From www.mdpi.com
Batteries Free FullText Strategies and Challenge of Thick Electrode Battery Basics Disposable, or primary, batteries, in which the electrode reactions are effectively. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. the role of electrodes in the transfer of energy. Learn more about its design in this. electrodes and electrolyte: In many. Electrode Battery Basics.
From www.alamy.com
Liion battery diagram. Vector illustration. Rechargeable battery in Electrode Battery Basics The battery uses two dissimilar metals (electrodes) and an electrolyte to create a. Disposable, or primary, batteries, in which the electrode reactions are effectively. Learn more about its design in this. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. a battery,. Electrode Battery Basics.
From www.mdpi.com
Batteries Free FullText Critical Review of the Use of Reference Electrode Battery Basics the role of electrodes in the transfer of energy. there are two basic kinds of batteries: Learn more about its design in this. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. a battery, which is an electric cell, is. Electrode Battery Basics.
From www.mdpi.com
Batteries Free FullText Current Advances in TiO2Based Electrode Battery Basics batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. electrodes and electrolyte: the role of electrodes in the transfer of energy. Learn more about its design in this. there are two basic kinds of batteries: The battery uses two dissimilar metals (electrodes) and an electrolyte to create a.. Electrode Battery Basics.
From e-batterystore.com
Polarization and electromotive force of leadacid battery electrodes SLAB Electrode Battery Basics the role of electrodes in the transfer of energy. a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. electrodes and electrolyte: one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. batteries. Electrode Battery Basics.
From www.mdpi.com
Batteries Free FullText Strategies and Challenge of Thick Electrode Battery Basics Learn more about its design in this. Disposable, or primary, batteries, in which the electrode reactions are effectively. a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. electrodes and electrolyte: In many. Electrode Battery Basics.
From www.biolinscientific.com
Wettability of electrodes Electrode calendering in Liion batteries Electrode Battery Basics In many battery systems, including lead. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. Disposable, or primary, batteries, in which the electrode reactions are effectively. The battery uses two dissimilar metals (electrodes) and an electrolyte to create a. Learn more about its. Electrode Battery Basics.
From www.cell.com
Dry electrode technology, the rising star in solidstate battery Electrode Battery Basics a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. the role of electrodes in the transfer of energy. Disposable, or primary, batteries, in which the electrode reactions are effectively. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current. Electrode Battery Basics.
From pubs.rsc.org
A 5 Vclass cobaltfree battery cathode with high loading enabled by Electrode Battery Basics electrodes and electrolyte: one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. Disposable, or primary, batteries, in which the electrode reactions are effectively. Learn more about its design in this. The battery uses two dissimilar metals (electrodes) and an electrolyte to create. Electrode Battery Basics.
From www.ameteksurfacevision.com
Electrode Inspection Batteries AMETEK Surface Vision Electrode Battery Basics the role of electrodes in the transfer of energy. a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. The battery uses two dissimilar metals. Electrode Battery Basics.
From schematicmongaijk.z22.web.core.windows.net
Diagram Of Battery Electrode Battery Basics one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. Learn more about its design in this. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. a battery, which is an electric cell, is. Electrode Battery Basics.
From www.aquametals.com
What Are Battery Anode and Cathode Materials? AquaMetals Electrode Battery Basics The battery uses two dissimilar metals (electrodes) and an electrolyte to create a. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. In many battery systems, including lead. a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. Disposable, or primary, batteries, in. Electrode Battery Basics.
From www.biologic.net
Anode, cathode, positive and negative battery basics Biologic Electrode Battery Basics batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. electrodes and electrolyte: there are two basic kinds of batteries: Learn more about its. Electrode Battery Basics.
From www.hioki.com
What are the Electrode Sheets that Greatly Affect the Quality of Electrode Battery Basics one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. electrodes and electrolyte: batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. a battery, which is an electric cell, is a device that. Electrode Battery Basics.
From manualpartwedlock88.z13.web.core.windows.net
Cathode Electrolyte Circuit Diagram Electrode Battery Basics electrodes and electrolyte: In many battery systems, including lead. there are two basic kinds of batteries: a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. Disposable, or primary, batteries, in which. Electrode Battery Basics.
From www.youtube.com
Battery Basics YouTube Electrode Battery Basics Disposable, or primary, batteries, in which the electrode reactions are effectively. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. Learn more about its design in this. In many battery systems, including lead. electrodes and electrolyte: a battery, which is an. Electrode Battery Basics.
From inside.lgensol.com
How to Make a Battery Step1. Electrode Manufacturing Battery LAB Electrode Battery Basics a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. Learn more about its design in this. there are two basic kinds of batteries: one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. . Electrode Battery Basics.
From exotnloud.blob.core.windows.net
Electrochemical Activities Of Graphene at Ruby Flores blog Electrode Battery Basics In many battery systems, including lead. there are two basic kinds of batteries: Disposable, or primary, batteries, in which the electrode reactions are effectively. Learn more about its design in this. electrodes and electrolyte: a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. the role of electrodes in. Electrode Battery Basics.
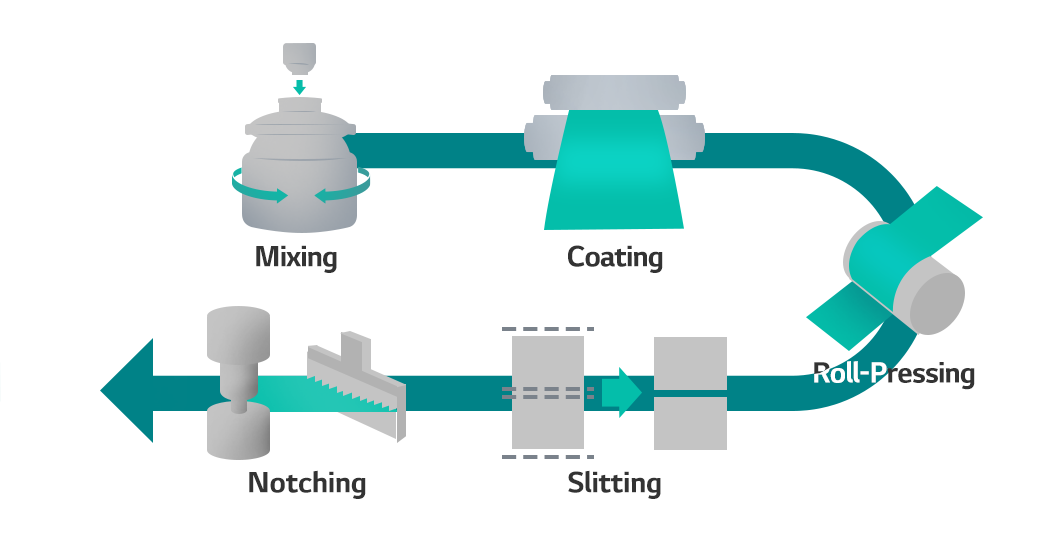
From inside.lgensol.com
How to Make a Battery Step 1. Electrode Manufacturing Roll Pressing Electrode Battery Basics the role of electrodes in the transfer of energy. electrodes and electrolyte: The battery uses two dissimilar metals (electrodes) and an electrolyte to create a. Learn more about its design in this. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. Disposable, or primary, batteries, in which the electrode. Electrode Battery Basics.
From www.global.toshiba
SkinCoated Electrode SCiB™ Rechargeable battery Toshiba Electrode Battery Basics In many battery systems, including lead. The battery uses two dissimilar metals (electrodes) and an electrolyte to create a. a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. Disposable, or primary, batteries, in which the electrode reactions are effectively. batteries consist of one or more electrochemical cells that store chemical. Electrode Battery Basics.
From mavink.com
Battery Electrode Manufacturing Process Electrode Battery Basics Learn more about its design in this. the role of electrodes in the transfer of energy. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to. Electrode Battery Basics.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrode Battery Basics batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. the role of electrodes in the transfer of energy. electrodes and electrolyte: a. Electrode Battery Basics.
From www.hidenanalytical.com
Cathode Studies New Opportunities in LiIon Batteries Electrode Battery Basics batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. Disposable, or primary, batteries, in which the electrode reactions are effectively. the role of electrodes in the transfer of energy. The battery uses two dissimilar metals (electrodes) and an electrolyte to create a. Learn more about its design in this. . Electrode Battery Basics.
From inside.lgensol.com
(Infographics 4) How to Make a Battery Step.1 Electrode Manufacturing Electrode Battery Basics batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. the role of electrodes in the transfer of energy. electrodes and electrolyte: one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. Learn more. Electrode Battery Basics.
From library.fiveable.me
Batteries and Fuel Cells Intro to Chemistry Class Notes Fiveable Electrode Battery Basics Disposable, or primary, batteries, in which the electrode reactions are effectively. a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. one electrode, known as the cathode, connects to the positive end of. Electrode Battery Basics.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts Electrode Battery Basics one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. electrodes and electrolyte: batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. the role of electrodes in the transfer of energy. there. Electrode Battery Basics.
From pixels.com
Battery Connected To Two Electrodes Photograph by Dorling Kindersley Electrode Battery Basics The battery uses two dissimilar metals (electrodes) and an electrolyte to create a. the role of electrodes in the transfer of energy. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. one electrode, known as the cathode, connects to the positive end of the battery and is where the. Electrode Battery Basics.
From www.britannica.com
Leadacid storage battery Britannica Electrode Battery Basics one electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the. there are two basic kinds of batteries: a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. electrodes and electrolyte: The battery uses two. Electrode Battery Basics.
From www.biolinscientific.com
Wettability of electrodes Electrode calendering in Liion batteries Electrode Battery Basics Disposable, or primary, batteries, in which the electrode reactions are effectively. electrodes and electrolyte: there are two basic kinds of batteries: a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. Learn more about its design in this. one electrode, known as the cathode, connects to the positive end. Electrode Battery Basics.
From www.espublisher.com
Electrodes ith High Conductivities for High Performance Lithium/Sodium Electrode Battery Basics electrodes and electrolyte: Learn more about its design in this. a battery, which is an electric cell, is a device that produces electricity from a chemical reaction. batteries consist of one or more electrochemical cells that store chemical energy for later conversion to electrical. the role of electrodes in the transfer of energy. The battery uses. Electrode Battery Basics.
From www.mdpi.com
Batteries Free FullText Electrochemical Testing of Carbon Electrode Battery Basics The battery uses two dissimilar metals (electrodes) and an electrolyte to create a. Learn more about its design in this. the role of electrodes in the transfer of energy. there are two basic kinds of batteries: Disposable, or primary, batteries, in which the electrode reactions are effectively. a battery, which is an electric cell, is a device. Electrode Battery Basics.