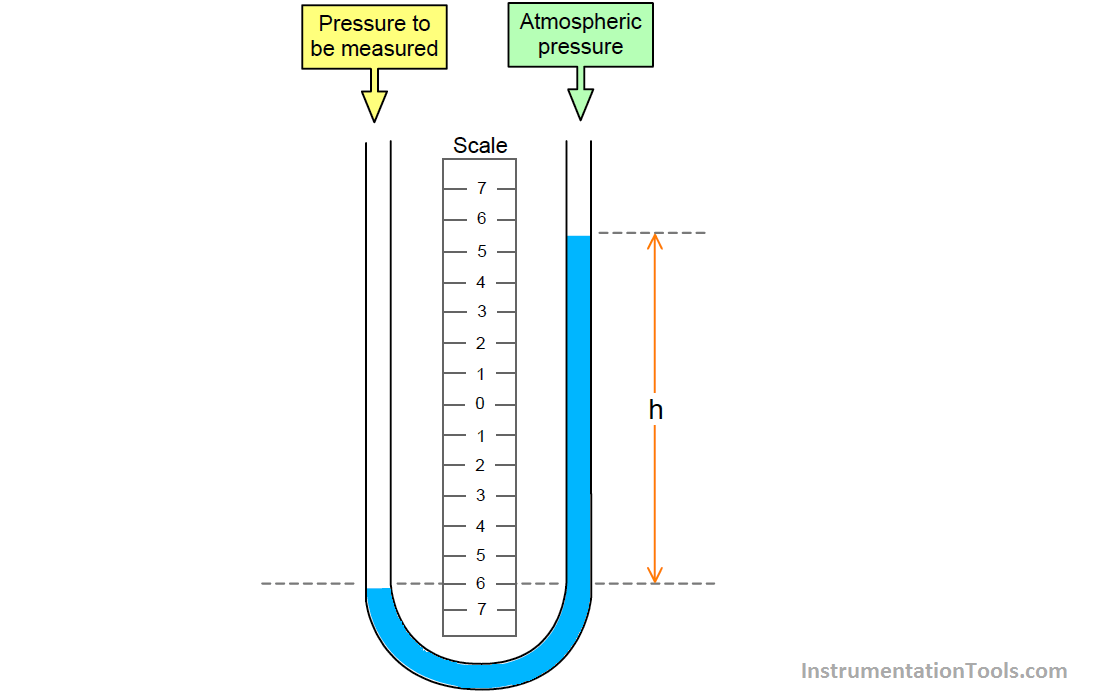
Example Of Manometer In Chemistry . The key feature of a manometer is. Manometers are typically used to measure pressure in a closed system of a gaseous matter. Barometers measure barometric pressure, but manometers measure the pressures of samples of gases contained in an apparatus. Plots pressure versus volume for a gas that obeys. There are two types of manometer, they are in u shape and filled with mercury. If one of the end is open to the. The equation below expresses boyle's law mathematically: Probably the most familiar example of a manometer is a sphygmomanometer, which is used to measure blood pressure. C is a constant unique to the temperature and mass of gas involved. Manometers are used for measure pressure of gas in closed container. The liquid in the manometer is mercury. A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. Assuming atmospheric pressure is 29.92.
from instrumentationtools.com
C is a constant unique to the temperature and mass of gas involved. The liquid in the manometer is mercury. There are two types of manometer, they are in u shape and filled with mercury. The key feature of a manometer is. Assuming atmospheric pressure is 29.92. Probably the most familiar example of a manometer is a sphygmomanometer, which is used to measure blood pressure. A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. Manometers are used for measure pressure of gas in closed container. Manometers are typically used to measure pressure in a closed system of a gaseous matter. If one of the end is open to the.
Utube Manometer Principle Inst Tools
Example Of Manometer In Chemistry The key feature of a manometer is. Manometers are typically used to measure pressure in a closed system of a gaseous matter. C is a constant unique to the temperature and mass of gas involved. The equation below expresses boyle's law mathematically: There are two types of manometer, they are in u shape and filled with mercury. Probably the most familiar example of a manometer is a sphygmomanometer, which is used to measure blood pressure. Plots pressure versus volume for a gas that obeys. Assuming atmospheric pressure is 29.92. A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. Manometers are used for measure pressure of gas in closed container. The liquid in the manometer is mercury. The key feature of a manometer is. Barometers measure barometric pressure, but manometers measure the pressures of samples of gases contained in an apparatus. If one of the end is open to the.
From theinstrumentguru.com
Primary sensing elements Example Of Manometer In Chemistry Manometers are used for measure pressure of gas in closed container. Plots pressure versus volume for a gas that obeys. Assuming atmospheric pressure is 29.92. The equation below expresses boyle's law mathematically: There are two types of manometer, they are in u shape and filled with mercury. The liquid in the manometer is mercury. If one of the end is. Example Of Manometer In Chemistry.
From mechanicalpost.site
What is manometer? types,uses and advantages The Mechanical post Example Of Manometer In Chemistry A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. C is a constant unique to the temperature and mass of gas involved. The liquid in the manometer is mercury. Manometers are used for measure pressure of gas in closed container. Barometers measure barometric pressure, but. Example Of Manometer In Chemistry.
From www.slideserve.com
PPT General Chemistry PowerPoint Presentation, free download ID5940887 Example Of Manometer In Chemistry Manometers are typically used to measure pressure in a closed system of a gaseous matter. Assuming atmospheric pressure is 29.92. Plots pressure versus volume for a gas that obeys. C is a constant unique to the temperature and mass of gas involved. Manometers are used for measure pressure of gas in closed container. Barometers measure barometric pressure, but manometers measure. Example Of Manometer In Chemistry.
From instrumentationtools.com
Utube Manometer Principle Inst Tools Example Of Manometer In Chemistry Barometers measure barometric pressure, but manometers measure the pressures of samples of gases contained in an apparatus. There are two types of manometer, they are in u shape and filled with mercury. The key feature of a manometer is. Manometers are used for measure pressure of gas in closed container. C is a constant unique to the temperature and mass. Example Of Manometer In Chemistry.
From www.youtube.com
Manometer Example YouTube Example Of Manometer In Chemistry The key feature of a manometer is. Assuming atmospheric pressure is 29.92. C is a constant unique to the temperature and mass of gas involved. A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. Probably the most familiar example of a manometer is a sphygmomanometer,. Example Of Manometer In Chemistry.
From www.youtube.com
Rise And Fall Of Liquid In U Shaped Manometer Tube SHM Example Example Of Manometer In Chemistry Assuming atmospheric pressure is 29.92. The key feature of a manometer is. C is a constant unique to the temperature and mass of gas involved. Plots pressure versus volume for a gas that obeys. There are two types of manometer, they are in u shape and filled with mercury. Manometers are used for measure pressure of gas in closed container.. Example Of Manometer In Chemistry.
From www.youtube.com
Manometer Example YouTube Example Of Manometer In Chemistry A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. The equation below expresses boyle's law mathematically: Manometers are used for measure pressure of gas in closed container. The key feature of a manometer is. C is a constant unique to the temperature and mass of. Example Of Manometer In Chemistry.
From www.youtube.com
How to solve manometer problems YouTube Example Of Manometer In Chemistry If one of the end is open to the. The key feature of a manometer is. The liquid in the manometer is mercury. C is a constant unique to the temperature and mass of gas involved. Plots pressure versus volume for a gas that obeys. Barometers measure barometric pressure, but manometers measure the pressures of samples of gases contained in. Example Of Manometer In Chemistry.
From www.youtube.com
Introductory Fluid Mechanics L5 p2 Example Manometer YouTube Example Of Manometer In Chemistry Manometers are used for measure pressure of gas in closed container. Manometers are typically used to measure pressure in a closed system of a gaseous matter. A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. The liquid in the manometer is mercury. C is a. Example Of Manometer In Chemistry.
From www.chemicalslearning.com
Types of Manometers, and Working Principle Example Of Manometer In Chemistry A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. The equation below expresses boyle's law mathematically: Manometers are typically used to measure pressure in a closed system of a gaseous matter. C is a constant unique to the temperature and mass of gas involved. The. Example Of Manometer In Chemistry.
From www.slideserve.com
PPT Chapter 5 Gases PowerPoint Presentation, free download ID6815320 Example Of Manometer In Chemistry Probably the most familiar example of a manometer is a sphygmomanometer, which is used to measure blood pressure. Manometers are used for measure pressure of gas in closed container. Barometers measure barometric pressure, but manometers measure the pressures of samples of gases contained in an apparatus. There are two types of manometer, they are in u shape and filled with. Example Of Manometer In Chemistry.
From byjus.com
A manometer consists of a U shaped tube containing water. One side is Example Of Manometer In Chemistry Manometers are typically used to measure pressure in a closed system of a gaseous matter. Assuming atmospheric pressure is 29.92. There are two types of manometer, they are in u shape and filled with mercury. The liquid in the manometer is mercury. Plots pressure versus volume for a gas that obeys. The key feature of a manometer is. Probably the. Example Of Manometer In Chemistry.
From www.slideserve.com
PPT AP Chemistry Unit 2 PowerPoint Presentation, free download ID Example Of Manometer In Chemistry C is a constant unique to the temperature and mass of gas involved. Manometers are used for measure pressure of gas in closed container. Manometers are typically used to measure pressure in a closed system of a gaseous matter. If one of the end is open to the. Barometers measure barometric pressure, but manometers measure the pressures of samples of. Example Of Manometer In Chemistry.
From automationforum.co
Explain the function of Manometer and it's types in detail. Example Of Manometer In Chemistry Assuming atmospheric pressure is 29.92. Barometers measure barometric pressure, but manometers measure the pressures of samples of gases contained in an apparatus. Probably the most familiar example of a manometer is a sphygmomanometer, which is used to measure blood pressure. There are two types of manometer, they are in u shape and filled with mercury. The liquid in the manometer. Example Of Manometer In Chemistry.
From eduinput.com
ManometerWorking, Types, And Applications Example Of Manometer In Chemistry Manometers are used for measure pressure of gas in closed container. Probably the most familiar example of a manometer is a sphygmomanometer, which is used to measure blood pressure. Manometers are typically used to measure pressure in a closed system of a gaseous matter. Plots pressure versus volume for a gas that obeys. A manometer is a device similar to. Example Of Manometer In Chemistry.
From sciencephotogallery.com
Manometer 1 by Science Photo Library Example Of Manometer In Chemistry The liquid in the manometer is mercury. C is a constant unique to the temperature and mass of gas involved. Plots pressure versus volume for a gas that obeys. Assuming atmospheric pressure is 29.92. Manometers are typically used to measure pressure in a closed system of a gaseous matter. If one of the end is open to the. Probably the. Example Of Manometer In Chemistry.
From www.chemicalslearning.com
Types of Manometers, and Working Principle Example Of Manometer In Chemistry A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. If one of the end is open to the. Manometers are used for measure pressure of gas in closed container. The equation below expresses boyle's law mathematically: Barometers measure barometric pressure, but manometers measure the pressures. Example Of Manometer In Chemistry.
From www.engineeringclicks.com
Manometer types and working principle EngineeringClicks Example Of Manometer In Chemistry If one of the end is open to the. Manometers are used for measure pressure of gas in closed container. Manometers are typically used to measure pressure in a closed system of a gaseous matter. Assuming atmospheric pressure is 29.92. C is a constant unique to the temperature and mass of gas involved. There are two types of manometer, they. Example Of Manometer In Chemistry.
From www.slideserve.com
PPT Honors Chemistry PowerPoint Presentation, free download ID6508012 Example Of Manometer In Chemistry There are two types of manometer, they are in u shape and filled with mercury. Manometers are used for measure pressure of gas in closed container. Plots pressure versus volume for a gas that obeys. C is a constant unique to the temperature and mass of gas involved. Probably the most familiar example of a manometer is a sphygmomanometer, which. Example Of Manometer In Chemistry.
From www.youtube.com
Simple manometer example problem YouTube Example Of Manometer In Chemistry The key feature of a manometer is. The equation below expresses boyle's law mathematically: A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. Plots pressure versus volume for a gas that obeys. C is a constant unique to the temperature and mass of gas involved.. Example Of Manometer In Chemistry.
From www.chemistrytutorials.org
Measuring Pressure of Gas and Manometers with Examples Online Example Of Manometer In Chemistry Manometers are typically used to measure pressure in a closed system of a gaseous matter. Assuming atmospheric pressure is 29.92. A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. Probably the most familiar example of a manometer is a sphygmomanometer, which is used to measure. Example Of Manometer In Chemistry.
From www.clutchprep.com
Manometer Chemistry Video Clutch Prep Example Of Manometer In Chemistry Barometers measure barometric pressure, but manometers measure the pressures of samples of gases contained in an apparatus. If one of the end is open to the. Manometers are used for measure pressure of gas in closed container. The equation below expresses boyle's law mathematically: There are two types of manometer, they are in u shape and filled with mercury. C. Example Of Manometer In Chemistry.
From chemicalengineeringworld.com
Manometer Definition & Types Chemical Engineering World Example Of Manometer In Chemistry Probably the most familiar example of a manometer is a sphygmomanometer, which is used to measure blood pressure. C is a constant unique to the temperature and mass of gas involved. Assuming atmospheric pressure is 29.92. Plots pressure versus volume for a gas that obeys. Manometers are used for measure pressure of gas in closed container. A manometer is a. Example Of Manometer In Chemistry.
From www.youtube.com
Manometer Example manometer hydrostatic YouTube Example Of Manometer In Chemistry Probably the most familiar example of a manometer is a sphygmomanometer, which is used to measure blood pressure. The key feature of a manometer is. Assuming atmospheric pressure is 29.92. A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. The equation below expresses boyle's law. Example Of Manometer In Chemistry.
From megadepot.com
What is Manometer and How Does it Work Mega Depot Example Of Manometer In Chemistry Plots pressure versus volume for a gas that obeys. The equation below expresses boyle's law mathematically: A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. There are two types of manometer, they are in u shape and filled with mercury. Manometers are used for measure. Example Of Manometer In Chemistry.
From www.youtube.com
Thermodynamics Example Manometer Problem YouTube Example Of Manometer In Chemistry Probably the most familiar example of a manometer is a sphygmomanometer, which is used to measure blood pressure. Plots pressure versus volume for a gas that obeys. A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. If one of the end is open to the.. Example Of Manometer In Chemistry.
From www.engineeringchoice.com
What Is Manometer? Definition, Working, and Types Example Of Manometer In Chemistry There are two types of manometer, they are in u shape and filled with mercury. Manometers are used for measure pressure of gas in closed container. Assuming atmospheric pressure is 29.92. Manometers are typically used to measure pressure in a closed system of a gaseous matter. Probably the most familiar example of a manometer is a sphygmomanometer, which is used. Example Of Manometer In Chemistry.
From chemistrytalk.org
Barometers and Manometers ChemTalk Example Of Manometer In Chemistry Manometers are used for measure pressure of gas in closed container. Manometers are typically used to measure pressure in a closed system of a gaseous matter. The liquid in the manometer is mercury. There are two types of manometer, they are in u shape and filled with mercury. Probably the most familiar example of a manometer is a sphygmomanometer, which. Example Of Manometer In Chemistry.
From engineerexcel.com
Manometer Equation Calculate Pressure from a Manometer Reading Example Of Manometer In Chemistry The liquid in the manometer is mercury. Manometers are typically used to measure pressure in a closed system of a gaseous matter. The key feature of a manometer is. If one of the end is open to the. Plots pressure versus volume for a gas that obeys. There are two types of manometer, they are in u shape and filled. Example Of Manometer In Chemistry.
From elshareitforfree.blogspot.com
Science Concepts and Questions (K to 12) 2018 Example Of Manometer In Chemistry A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. Assuming atmospheric pressure is 29.92. Manometers are typically used to measure pressure in a closed system of a gaseous matter. Manometers are used for measure pressure of gas in closed container. The liquid in the manometer. Example Of Manometer In Chemistry.
From www.youtube.com
Simple Manometer Example Problem 2 YouTube Example Of Manometer In Chemistry The equation below expresses boyle's law mathematically: Probably the most familiar example of a manometer is a sphygmomanometer, which is used to measure blood pressure. Manometers are typically used to measure pressure in a closed system of a gaseous matter. There are two types of manometer, they are in u shape and filled with mercury. Manometers are used for measure. Example Of Manometer In Chemistry.
From www.engineeringchoice.com
What Is Manometer? Definition, Working, And Types Engineering Choice Example Of Manometer In Chemistry Assuming atmospheric pressure is 29.92. A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. Manometers are typically used to measure pressure in a closed system of a gaseous matter. The liquid in the manometer is mercury. Plots pressure versus volume for a gas that obeys.. Example Of Manometer In Chemistry.
From www.slideserve.com
PPT Unit 6 Gases & The Molecular Theory PowerPoint Example Of Manometer In Chemistry If one of the end is open to the. Barometers measure barometric pressure, but manometers measure the pressures of samples of gases contained in an apparatus. C is a constant unique to the temperature and mass of gas involved. There are two types of manometer, they are in u shape and filled with mercury. Manometers are used for measure pressure. Example Of Manometer In Chemistry.
From automationforum.co
Explain the function of Manometer and it's types in detail. Example Of Manometer In Chemistry The liquid in the manometer is mercury. There are two types of manometer, they are in u shape and filled with mercury. The equation below expresses boyle's law mathematically: C is a constant unique to the temperature and mass of gas involved. Probably the most familiar example of a manometer is a sphygmomanometer, which is used to measure blood pressure.. Example Of Manometer In Chemistry.
From chemicalengineeringworld.com
Manometer Definition & Types Chemical Engineering World Example Of Manometer In Chemistry The key feature of a manometer is. Barometers measure barometric pressure, but manometers measure the pressures of samples of gases contained in an apparatus. Plots pressure versus volume for a gas that obeys. There are two types of manometer, they are in u shape and filled with mercury. Manometers are used for measure pressure of gas in closed container. Assuming. Example Of Manometer In Chemistry.