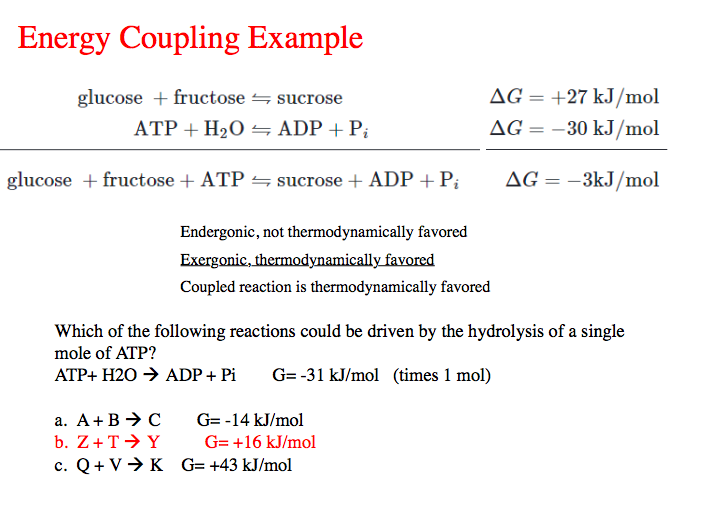
Coupling Reaction Example . One simple example of the coupling of reaction is the decomposition of calcium carbonate: Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. First law of thermodynamics introduction. Second law of thermodynamics and entropy. An organism’s metabolism is the sum total of all of these various reactions. Unfavorable reactions occur when they are coupled to. 20 rows in organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined.
from wasfa-hd.blogspot.com
One simple example of the coupling of reaction is the decomposition of calcium carbonate: A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. 20 rows in organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. Unfavorable reactions occur when they are coupled to. Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. An organism’s metabolism is the sum total of all of these various reactions. Second law of thermodynamics and entropy. First law of thermodynamics introduction. A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined.
Atp To Adp Plus Pi Is An Example Of What Reaction Wasfa Blog
Coupling Reaction Example A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. First law of thermodynamics introduction. Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. 20 rows in organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. Unfavorable reactions occur when they are coupled to. Second law of thermodynamics and entropy. One simple example of the coupling of reaction is the decomposition of calcium carbonate: A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. An organism’s metabolism is the sum total of all of these various reactions. A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined.
From www.synarchive.com
Hiyama Coupling Coupling Reaction Example 20 rows in organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. Second law of thermodynamics and entropy. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. Caco3 (s) ⇌ cao (s) + co2. Coupling Reaction Example.
From wasfa-hd.blogspot.com
Atp To Adp Plus Pi Is An Example Of What Reaction Wasfa Blog Coupling Reaction Example An organism’s metabolism is the sum total of all of these various reactions. A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined. 20 rows in organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together.. Coupling Reaction Example.
From www.sliderbase.com
Presentation Chemistry Coupling Reaction Example Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined. Second law of thermodynamics and entropy. Unfavorable reactions occur when they are coupled to. One simple example of the coupling. Coupling Reaction Example.
From scienceinfo.com
Coupled ReactionThermodynamics Explanation, Example Coupling Reaction Example One simple example of the coupling of reaction is the decomposition of calcium carbonate: Second law of thermodynamics and entropy. A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined. First law of thermodynamics introduction. Caco3 (s) ⇌ cao (s) + co2 (g) δgo. Coupling Reaction Example.
From www.slideserve.com
PPT Palladium Assisted Coupling Reactions PowerPoint Presentation Coupling Reaction Example An organism’s metabolism is the sum total of all of these various reactions. A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined. Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. A coupling reaction in organic chemistry is. Coupling Reaction Example.
From www.wizeprep.com
Coupled Reactions Wize University Biology Textbook Wizeprep Coupling Reaction Example Unfavorable reactions occur when they are coupled to. First law of thermodynamics introduction. An organism’s metabolism is the sum total of all of these various reactions. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. A reaction where the the free energy of. Coupling Reaction Example.
From www.biologyonline.com
Energy coupling Definition and Examples Biology Online Dictionary Coupling Reaction Example A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined. One simple example of the coupling of reaction is the decomposition of calcium carbonate: Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. Unfavorable reactions occur when they are. Coupling Reaction Example.
From sciencevivid.com
Metabolism Definition, Purposes, Types, Regulations, Microbiota Coupling Reaction Example Second law of thermodynamics and entropy. One simple example of the coupling of reaction is the decomposition of calcium carbonate: An organism’s metabolism is the sum total of all of these various reactions. 20 rows in organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. A reaction where the the free. Coupling Reaction Example.
From sciencenotes.org
Endergonic vs Exergonic Reactions and Examples Coupling Reaction Example A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined. Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with. Coupling Reaction Example.
From pharmacyscope.com
Diazo Coupling Reaction Pharmacy Scope Coupling Reaction Example Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. One simple example of the coupling of reaction is the decomposition of calcium carbonate: Second law of thermodynamics and entropy. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst.. Coupling Reaction Example.
From www.numerade.com
SOLVED Label the figure to identify an ATPcoupled reaction Coupling Reaction Example Second law of thermodynamics and entropy. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. Unfavorable reactions occur when they are coupled to. 20 rows in organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded. Coupling Reaction Example.
From www.flexiprep.com
Chemistry Class 12 NCERT Solutions Chapter 13 Amines Part 9 FlexiPrep Coupling Reaction Example Second law of thermodynamics and entropy. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. First law of thermodynamics introduction. A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one,. Coupling Reaction Example.
From chemistry-reaction.com
Stille Coupling Reaction Mechanism with Application Coupling Reaction Example First law of thermodynamics introduction. Unfavorable reactions occur when they are coupled to. Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. An organism’s metabolism is the sum total of all of these various reactions. 20 rows in organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded. Coupling Reaction Example.
From www.youtube.com
ATP and Coupled Reactions.mp4 YouTube Coupling Reaction Example An organism’s metabolism is the sum total of all of these various reactions. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. First law of thermodynamics introduction. Second law of thermodynamics and entropy. One simple example of the coupling of reaction is the. Coupling Reaction Example.
From www.slideserve.com
PPT Chapter 8 An Introduction to Metabolism PowerPoint Presentation Coupling Reaction Example Second law of thermodynamics and entropy. 20 rows in organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. Unfavorable reactions occur when they are coupled to. Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. A coupling reaction in organic chemistry is a general term for. Coupling Reaction Example.
From www.youtube.com
Let's Think about reaction coupling YouTube Coupling Reaction Example Second law of thermodynamics and entropy. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined. Caco3 (s). Coupling Reaction Example.
From chemistry-reaction.com
Suzuki crosscoupling Reaction Examples Mechanism Application Coupling Reaction Example Unfavorable reactions occur when they are coupled to. One simple example of the coupling of reaction is the decomposition of calcium carbonate: Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. First law of thermodynamics introduction. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together. Coupling Reaction Example.
From nrochemistry.com
Negishi Coupling Coupling Reaction Example A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined. Unfavorable reactions occur when they are coupled to. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst.. Coupling Reaction Example.
From www.coursehero.com
[Solved] .3 what is diazo coupling reaction explains with example and Coupling Reaction Example Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. Unfavorable reactions occur when they are coupled to. Second law of thermodynamics and entropy. One simple example of the coupling of reaction is the decomposition of calcium carbonate: An organism’s metabolism is the sum total of all of these various reactions. 20 rows in organic chemistry,. Coupling Reaction Example.
From chemistnotes.com
Suzuki reaction easy mechanism,application Chemistry Notes Coupling Reaction Example First law of thermodynamics introduction. Second law of thermodynamics and entropy. Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. One simple example of the coupling of reaction is the decomposition of calcium carbonate: 20 rows in organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together.. Coupling Reaction Example.
From nrochemistry.com
Heck Coupling Coupling Reaction Example A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined. 20 rows in organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. First law of thermodynamics introduction. A coupling reaction in organic chemistry is a. Coupling Reaction Example.
From ar.inspiredpencil.com
Coupled Reactions Coupling Reaction Example A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined. An organism’s metabolism is the sum total of all of these various reactions. Second law of thermodynamics and entropy. First law of thermodynamics introduction. Unfavorable reactions occur when they are coupled to. A coupling. Coupling Reaction Example.
From www.youtube.com
Stille Coupling Reaction Mechanism YouTube Coupling Reaction Example Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. An organism’s metabolism is the sum total of all of these various reactions. Unfavorable reactions occur when they are coupled to. One simple example of the coupling of reaction is the decomposition of calcium carbonate: First law of thermodynamics introduction. A coupling reaction in organic chemistry. Coupling Reaction Example.
From www.synarchive.com
Ullmann Coupling Coupling Reaction Example A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined. Second law of thermodynamics and entropy. One simple example of the coupling of reaction is the decomposition of calcium carbonate: Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol.. Coupling Reaction Example.
From www.youtube.com
Coupled reactions Applications of thermodynamics AP Chemistry Coupling Reaction Example Second law of thermodynamics and entropy. Unfavorable reactions occur when they are coupled to. One simple example of the coupling of reaction is the decomposition of calcium carbonate: First law of thermodynamics introduction. An organism’s metabolism is the sum total of all of these various reactions. 20 rows in organic chemistry, a coupling reaction is a type of reaction in. Coupling Reaction Example.
From chemistry-reaction.com
Suzuki crosscoupling Reaction Examples Mechanism Application Coupling Reaction Example Second law of thermodynamics and entropy. 20 rows in organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. One simple example of the coupling of reaction is the decomposition of calcium carbonate: A reaction where the the. Coupling Reaction Example.
From tammy.ai
Understanding Cellular Metabolism From Glucose Phosphorylation to Coupling Reaction Example Second law of thermodynamics and entropy. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. 20 rows in organic chemistry, a coupling reaction is a type of reaction in. Coupling Reaction Example.
From golifescience.com
Coupling Reactions Importance in Biological Systems Coupling Reaction Example Second law of thermodynamics and entropy. Unfavorable reactions occur when they are coupled to. Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. 20 rows in organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. A reaction where the the free energy of a thermodynamically favorable. Coupling Reaction Example.
From www.youtube.com
Energy Coupling YouTube Coupling Reaction Example An organism’s metabolism is the sum total of all of these various reactions. Second law of thermodynamics and entropy. One simple example of the coupling of reaction is the decomposition of calcium carbonate: First law of thermodynamics introduction. Unfavorable reactions occur when they are coupled to. A reaction where the the free energy of a thermodynamically favorable transformation, such as. Coupling Reaction Example.
From iubmb.onlinelibrary.wiley.com
Coupled reactions versus connected reactions Coupling concepts with Coupling Reaction Example A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined. One simple example of the coupling of reaction is the decomposition of calcium carbonate: Unfavorable reactions occur when they are coupled to. A coupling reaction in organic chemistry is a general term for various. Coupling Reaction Example.
From www.mdpi.com
Reactions Free FullText CN, CO and CS UllmannType Coupling Coupling Reaction Example Unfavorable reactions occur when they are coupled to. Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. 20 rows in organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. An organism’s metabolism is the sum total of all of these various reactions. First law of thermodynamics. Coupling Reaction Example.
From www.pinterest.co.uk
Mechanism of coupling reaction. Reactions, Organic chemistry, Chemistry Coupling Reaction Example First law of thermodynamics introduction. Unfavorable reactions occur when they are coupled to. A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined. Second law of thermodynamics and entropy. A coupling reaction in organic chemistry is a general term for various reactions where two. Coupling Reaction Example.
From ppt-online.org
Organic Compounds презентация онлайн Coupling Reaction Example A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined. An organism’s metabolism is the sum total of all of these various reactions. Unfavorable reactions occur when they are coupled to. Second law of thermodynamics and entropy. A coupling reaction in organic chemistry is. Coupling Reaction Example.
From www.youtube.com
Coupled Reactions YouTube Coupling Reaction Example Second law of thermodynamics and entropy. A reaction where the the free energy of a thermodynamically favorable transformation, such as the hydrolysis of atp, and a thermodynamically unfavorable one, are mechanistically joined. First law of thermodynamics introduction. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of. Coupling Reaction Example.
From www.researchgate.net
Reaction schemes a SM coupling and b Heck coupling Download Coupling Reaction Example An organism’s metabolism is the sum total of all of these various reactions. Second law of thermodynamics and entropy. First law of thermodynamics introduction. 20 rows in organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. Caco3 (s) ⇌ cao (s) + co2 (g) δgo = 130.40 kj / mol. A. Coupling Reaction Example.