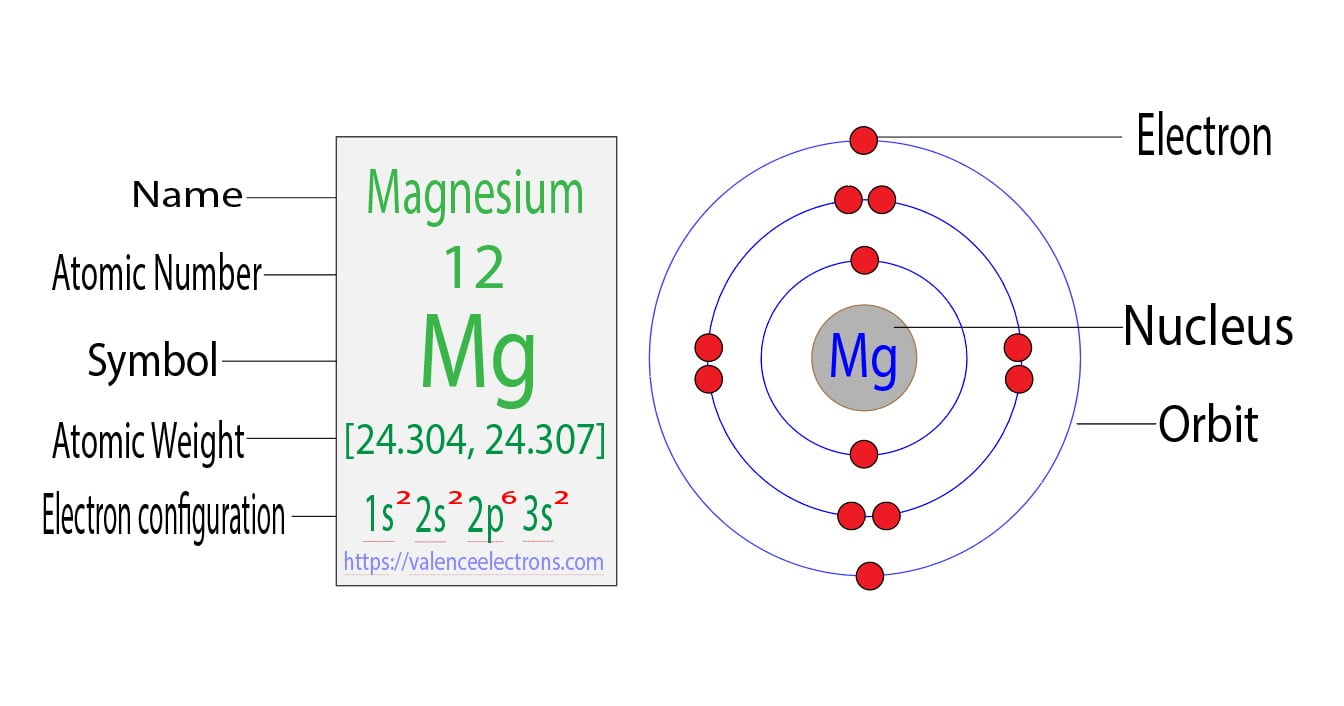
Magnesium Electron Behavior . Mastering the electron configuration of magnesium is essential for understanding its chemical behavior. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The shorthand electron configuration (or noble gas configuration) as well as. In the case of a metal it really doesn't make sense to say magnesium has no unpaired electrons, there are valence electrons. Electron configuration of magnesium is [ne] 3s2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². 93 rows updated on may 19, 2024. One of the key characteristics of magnesium is its electron shell diagram, which provides a visual representation of how the electrons are. Magnesium occurs naturally only in combination with other elements, where it invariably has a +2 oxidation state. Electron configuration chart of all elements is mentioned in the table below. Magnesium has two valence electrons in the 3s orbital. Possible oxidation states are +2. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons).
from valenceelectrons.com
In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Possible oxidation states are +2. One of the key characteristics of magnesium is its electron shell diagram, which provides a visual representation of how the electrons are. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s orbital. Electron configuration chart of all elements is mentioned in the table below. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Magnesium occurs naturally only in combination with other elements, where it invariably has a +2 oxidation state. The shorthand electron configuration (or noble gas configuration) as well as. In the case of a metal it really doesn't make sense to say magnesium has no unpaired electrons, there are valence electrons.
How to Write the Electron Configuration for Magnesium (Mg)?
Magnesium Electron Behavior One of the key characteristics of magnesium is its electron shell diagram, which provides a visual representation of how the electrons are. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). In the case of a metal it really doesn't make sense to say magnesium has no unpaired electrons, there are valence electrons. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². One of the key characteristics of magnesium is its electron shell diagram, which provides a visual representation of how the electrons are. Magnesium has two valence electrons in the 3s orbital. Magnesium occurs naturally only in combination with other elements, where it invariably has a +2 oxidation state. The shorthand electron configuration (or noble gas configuration) as well as. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Mastering the electron configuration of magnesium is essential for understanding its chemical behavior. Possible oxidation states are +2. Electron configuration chart of all elements is mentioned in the table below. 93 rows updated on may 19, 2024. Electron configuration of magnesium is [ne] 3s2.
From wiringmanualcaponising.z21.web.core.windows.net
Correct Electron Dot Diagram For Magnesium Magnesium Electron Behavior Magnesium occurs naturally only in combination with other elements, where it invariably has a +2 oxidation state. Magnesium has two valence electrons in the 3s orbital. In the case of a metal it really doesn't make sense to say magnesium has no unpaired electrons, there are valence electrons. In order to write the mg electron configuration we first need to. Magnesium Electron Behavior.
From www.shutterstock.com
Magnesium Atom. Diagram Representation Of The Element Magnesium Magnesium Electron Behavior Possible oxidation states are +2. Magnesium occurs naturally only in combination with other elements, where it invariably has a +2 oxidation state. One of the key characteristics of magnesium is its electron shell diagram, which provides a visual representation of how the electrons are. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Mastering the electron configuration of magnesium. Magnesium Electron Behavior.
From www.pinterest.com
Which Magnesium to Take Start by avoiding magnesium oxide, hydroxide Magnesium Electron Behavior The shorthand electron configuration (or noble gas configuration) as well as. Magnesium occurs naturally only in combination with other elements, where it invariably has a +2 oxidation state. Mastering the electron configuration of magnesium is essential for understanding its chemical behavior. Possible oxidation states are +2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². You may assume the. Magnesium Electron Behavior.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Electron Behavior In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Magnesium has two valence electrons in the 3s orbital. Possible oxidation states are +2. 93 rows updated on may 19, 2024. Electron configuration chart of all elements is mentioned in the table below. Magnesium occurs. Magnesium Electron Behavior.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Electron Behavior Electron configuration of magnesium is [ne] 3s2. Electron configuration chart of all elements is mentioned in the table below. Possible oxidation states are +2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s orbital. You may assume the valences of the chemical elements—the number of electrons with which an atom will. Magnesium Electron Behavior.
From www.dreamstime.com
Atom Of Magnesium With Detailed Core And Its 12 Electrons Stock Magnesium Electron Behavior In the case of a metal it really doesn't make sense to say magnesium has no unpaired electrons, there are valence electrons. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Electron configuration chart of all elements is mentioned in the table below. Mastering the electron configuration. Magnesium Electron Behavior.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Electron Behavior Electron configuration chart of all elements is mentioned in the table below. Possible oxidation states are +2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². 93 rows updated on may 19, 2024. Magnesium occurs naturally only in combination with other elements, where it invariably has a +2 oxidation state. Mastering the electron configuration of magnesium is essential for. Magnesium Electron Behavior.
From loegrhpel.blob.core.windows.net
Magnesium Electron Configuration Full at Ronny Robb blog Magnesium Electron Behavior Electron configuration of magnesium is [ne] 3s2. 93 rows updated on may 19, 2024. The shorthand electron configuration (or noble gas configuration) as well as. Possible oxidation states are +2. Magnesium has two valence electrons in the 3s orbital. In the case of a metal it really doesn't make sense to say magnesium has no unpaired electrons, there are valence. Magnesium Electron Behavior.
From edurev.in
A) write the electron dot structure for sodium,oxygen,magnesium b) show Magnesium Electron Behavior Magnesium has two valence electrons in the 3s orbital. Possible oxidation states are +2. Electron configuration chart of all elements is mentioned in the table below. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The shorthand electron configuration (or noble gas configuration) as well as. The. Magnesium Electron Behavior.
From montessorimuddle.org
subatomic particles Montessori Muddle Magnesium Electron Behavior Magnesium occurs naturally only in combination with other elements, where it invariably has a +2 oxidation state. Magnesium has two valence electrons in the 3s orbital. Mastering the electron configuration of magnesium is essential for understanding its chemical behavior. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom. Magnesium Electron Behavior.
From www.fity.club
Magnesium Electron Configuration Youtube Magnesium Electron Behavior In the case of a metal it really doesn't make sense to say magnesium has no unpaired electrons, there are valence electrons. Electron configuration chart of all elements is mentioned in the table below. One of the key characteristics of magnesium is its electron shell diagram, which provides a visual representation of how the electrons are. Magnesium has two valence. Magnesium Electron Behavior.
From yeseniadesnhday.blogspot.com
Electron Arrangement of Magnesium Magnesium Electron Behavior The shorthand electron configuration (or noble gas configuration) as well as. Magnesium occurs naturally only in combination with other elements, where it invariably has a +2 oxidation state. Electron configuration chart of all elements is mentioned in the table below. Electron configuration of magnesium is [ne] 3s2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². 93 rows updated. Magnesium Electron Behavior.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Behavior Electron configuration chart of all elements is mentioned in the table below. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². 93 rows updated on may 19, 2024. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Possible oxidation states are +2. Magnesium occurs naturally only. Magnesium Electron Behavior.
From sujatanutripharma.com
Magnesium Hydroxide Sujata Nutri Pharma Magnesium Electron Behavior 93 rows updated on may 19, 2024. Mastering the electron configuration of magnesium is essential for understanding its chemical behavior. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of. Magnesium Electron Behavior.
From www.pinterest.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Electron Behavior In the case of a metal it really doesn't make sense to say magnesium has no unpaired electrons, there are valence electrons. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. One of the key characteristics of magnesium is its electron shell diagram, which provides a visual. Magnesium Electron Behavior.
From www.alamy.com
Trimagnesium citrate hires stock photography and images Alamy Magnesium Electron Behavior One of the key characteristics of magnesium is its electron shell diagram, which provides a visual representation of how the electrons are. 93 rows updated on may 19, 2024. Magnesium has two valence electrons in the 3s orbital. Mastering the electron configuration of magnesium is essential for understanding its chemical behavior. Electron configuration chart of all elements is mentioned in. Magnesium Electron Behavior.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Behavior Mastering the electron configuration of magnesium is essential for understanding its chemical behavior. Magnesium has two valence electrons in the 3s orbital. One of the key characteristics of magnesium is its electron shell diagram, which provides a visual representation of how the electrons are. In order to write the mg electron configuration we first need to know the number of. Magnesium Electron Behavior.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Behavior Possible oxidation states are +2. One of the key characteristics of magnesium is its electron shell diagram, which provides a visual representation of how the electrons are. In the case of a metal it really doesn't make sense to say magnesium has no unpaired electrons, there are valence electrons. Electron configuration chart of all elements is mentioned in the table. Magnesium Electron Behavior.
From www.archyde.com
Magnesium Supplements Are Growing in Popularity Is It a Good Thing Magnesium Electron Behavior Magnesium has two valence electrons in the 3s orbital. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). 93 rows updated on may 19, 2024. One of the key characteristics of magnesium is its electron shell diagram, which provides a visual representation of how. Magnesium Electron Behavior.
From periodiske-system.dk
Magnesium Magnesium Electron Behavior The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The shorthand electron configuration (or noble gas configuration) as well as. Mastering the electron configuration of magnesium is essential for understanding its chemical behavior. One of the key characteristics of magnesium is its electron shell diagram, which provides a visual representation of how the electrons are. Possible oxidation states are. Magnesium Electron Behavior.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Electron Behavior In the case of a metal it really doesn't make sense to say magnesium has no unpaired electrons, there are valence electrons. Possible oxidation states are +2. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. One of the key characteristics of magnesium is its electron. Magnesium Electron Behavior.
From labeasy.blogspot.com
Magnesium hydroxide mixture B.P Magnesium Electron Behavior Electron configuration of magnesium is [ne] 3s2. The shorthand electron configuration (or noble gas configuration) as well as. Mastering the electron configuration of magnesium is essential for understanding its chemical behavior. Magnesium has two valence electrons in the 3s orbital. One of the key characteristics of magnesium is its electron shell diagram, which provides a visual representation of how the. Magnesium Electron Behavior.
From www.fity.club
Magnesium Electron Configuration Youtube Magnesium Electron Behavior Magnesium has two valence electrons in the 3s orbital. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Mastering the electron configuration of magnesium is essential for understanding its chemical behavior. Possible oxidation states. Magnesium Electron Behavior.
From www.fity.club
What Is The Orbital Diagram For Magnesium Quora Magnesium Electron Behavior 93 rows updated on may 19, 2024. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Magnesium occurs naturally only in combination with other elements, where it invariably has a +2 oxidation state. Magnesium has two valence electrons in the 3s orbital. Electron configuration chart of all. Magnesium Electron Behavior.
From loelhhatr.blob.core.windows.net
What Does Not Have Atoms at Courtney Galvez blog Magnesium Electron Behavior In the case of a metal it really doesn't make sense to say magnesium has no unpaired electrons, there are valence electrons. Magnesium occurs naturally only in combination with other elements, where it invariably has a +2 oxidation state. The shorthand electron configuration (or noble gas configuration) as well as. Possible oxidation states are +2. 93 rows updated on may. Magnesium Electron Behavior.
From nursehub.com
Electron Shells NurseHub Magnesium Electron Behavior Possible oxidation states are +2. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Electron configuration chart of all elements is mentioned in the table below. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom. Magnesium Electron Behavior.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Electron Behavior In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Electron configuration of magnesium is [ne] 3s2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium occurs naturally only in combination with other elements, where it invariably has a +2 oxidation state. One. Magnesium Electron Behavior.
From www.pinterest.com
Magnesium Electron Configuration Electron configuration, Electrons Magnesium Electron Behavior Magnesium has two valence electrons in the 3s orbital. In the case of a metal it really doesn't make sense to say magnesium has no unpaired electrons, there are valence electrons. 93 rows updated on may 19, 2024. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that.. Magnesium Electron Behavior.
From en.wikipedia.org
FileMagnesiumsulfide3Dionic.png Wikipedia Magnesium Electron Behavior The shorthand electron configuration (or noble gas configuration) as well as. Mastering the electron configuration of magnesium is essential for understanding its chemical behavior. 93 rows updated on may 19, 2024. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium occurs naturally only in combination with other elements, where it invariably has a +2 oxidation state. Possible oxidation. Magnesium Electron Behavior.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Magnesium Electron Behavior Magnesium has two valence electrons in the 3s orbital. The shorthand electron configuration (or noble gas configuration) as well as. One of the key characteristics of magnesium is its electron shell diagram, which provides a visual representation of how the electrons are. Possible oxidation states are +2. In order to write the mg electron configuration we first need to know. Magnesium Electron Behavior.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium Electron Behavior Electron configuration chart of all elements is mentioned in the table below. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Electron configuration of magnesium is [ne] 3s2. Mastering the electron configuration of magnesium is essential for understanding its chemical behavior. The shorthand electron configuration (or noble gas configuration) as well as. One of the key characteristics of magnesium. Magnesium Electron Behavior.
From www.revimage.org
Second Most Abundant Element In The Earth S Crust With 14 Neutrons Magnesium Electron Behavior Mastering the electron configuration of magnesium is essential for understanding its chemical behavior. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². One of the key characteristics of magnesium is its electron shell diagram, which provides a visual representation of how the electrons are. In the case of a metal it really doesn't make sense to say magnesium has. Magnesium Electron Behavior.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Behavior Possible oxidation states are +2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In the case of a metal it really doesn't make sense to say magnesium has no unpaired electrons, there are valence electrons. Magnesium occurs naturally only in combination with other elements, where it invariably has a +2 oxidation state. Electron configuration of magnesium is [ne]. Magnesium Electron Behavior.
From valenceelectrons.com
How to Find the Valence Electrons for Magnesium (Mg)? Magnesium Electron Behavior In the case of a metal it really doesn't make sense to say magnesium has no unpaired electrons, there are valence electrons. Mastering the electron configuration of magnesium is essential for understanding its chemical behavior. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons).. Magnesium Electron Behavior.
From www.nsf.gov
Multimedia Gallery Asymmetric Electron Behavior in Hightemperature Magnesium Electron Behavior One of the key characteristics of magnesium is its electron shell diagram, which provides a visual representation of how the electrons are. In the case of a metal it really doesn't make sense to say magnesium has no unpaired electrons, there are valence electrons. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Electron configuration chart of all elements. Magnesium Electron Behavior.