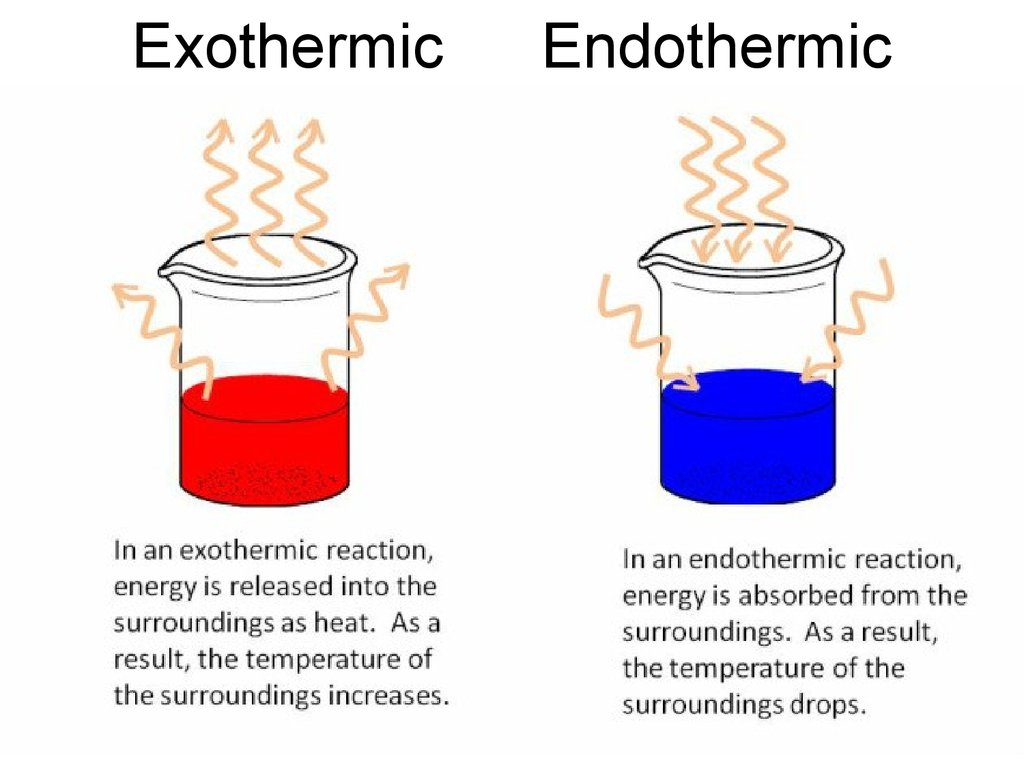
Endothermic Reaction Decrease Temperature . Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. When energy is absorbed in an endothermic reaction, the temperature decreases. The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become colder. Dissolution of ammonium nitrate in water, as in. When energy is taken in from the surroundings, this is called an endothermic close endothermic reaction in which energy is taken in. This shifts chemical equilibria toward the products or. A thermometer is used to detect the temperature decrease. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. A temperature change occurs when temperature is increased or decreased by the flow of heat. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets.
from en.ppt-online.org
A temperature change occurs when temperature is increased or decreased by the flow of heat. The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become colder. When energy is absorbed in an endothermic reaction, the temperature decreases. A thermometer is used to detect the temperature decrease. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets. This shifts chemical equilibria toward the products or. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. When energy is taken in from the surroundings, this is called an endothermic close endothermic reaction in which energy is taken in. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases.
Thermal Energy, Chemical Energy online presentation
Endothermic Reaction Decrease Temperature A thermometer is used to detect the temperature decrease. When energy is taken in from the surroundings, this is called an endothermic close endothermic reaction in which energy is taken in. A thermometer is used to detect the temperature decrease. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. This shifts chemical equilibria toward the products or. The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become colder. A temperature change occurs when temperature is increased or decreased by the flow of heat. Dissolution of ammonium nitrate in water, as in. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets. When energy is absorbed in an endothermic reaction, the temperature decreases.
From mmerevise.co.uk
Enthalpy Changes and Calorimetry MME Endothermic Reaction Decrease Temperature By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. When energy. Endothermic Reaction Decrease Temperature.
From lessoncampusspumier.z21.web.core.windows.net
Identify Endothermic And Exothermic Reactions Endothermic Reaction Decrease Temperature When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets. The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become colder. Thus, an endothermic reaction. Endothermic Reaction Decrease Temperature.
From general.chemistrysteps.com
Entropy Changes in the Surroundings Chemistry Steps Endothermic Reaction Decrease Temperature When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. This shifts chemical equilibria toward the products or. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets. When energy is absorbed in an endothermic reaction, the temperature decreases. When energy is. Endothermic Reaction Decrease Temperature.
From www.w3schools.blog
Factors affecting the rate of a reaction Temperature W3schools Endothermic Reaction Decrease Temperature The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become colder. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. This shifts chemical equilibria toward the products or. A temperature change occurs when temperature is increased or decreased by the flow of heat. When energy is absorbed. Endothermic Reaction Decrease Temperature.
From loebantdv.blob.core.windows.net
Why Does Equilibrium Vapor Pressure Increase With Temperature at Thomas Endothermic Reaction Decrease Temperature In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets. When energy is absorbed in an endothermic reaction, the temperature decreases. A thermometer is used to detect the temperature decrease. This shifts chemical equilibria toward the products or. A temperature change occurs when temperature is increased or. Endothermic Reaction Decrease Temperature.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review Endothermic Reaction Decrease Temperature Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. A temperature change occurs when temperature is increased or decreased by the flow of heat. A thermometer is used to detect the temperature decrease. When. Endothermic Reaction Decrease Temperature.
From www.slideserve.com
PPT Exothermic and endothermic reactions PowerPoint Presentation Endothermic Reaction Decrease Temperature By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Dissolution of ammonium nitrate in water, as in. The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become colder. This shifts chemical equilibria toward the products or. When energy is taken in from the surroundings, this is called an endothermic close. Endothermic Reaction Decrease Temperature.
From stock.adobe.com
Activation energy in endothermic and exothermic reactions. Stock Endothermic Reaction Decrease Temperature When energy is absorbed in an endothermic reaction, the temperature decreases. A temperature change occurs when temperature is increased or decreased by the flow of heat. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets. When energy is released in an exothermic reaction, the temperature of. Endothermic Reaction Decrease Temperature.
From mungfali.com
Exothermic Vs Endothermic Reaction Graph Endothermic Reaction Decrease Temperature This shifts chemical equilibria toward the products or. When energy is absorbed in an endothermic reaction, the temperature decreases. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets. Dissolution of ammonium. Endothermic Reaction Decrease Temperature.
From www.numerade.com
SOLVEDAn endothermic reaction causes the surroundings to condense Endothermic Reaction Decrease Temperature When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. When energy is absorbed in an endothermic reaction, the temperature decreases. A temperature change occurs when temperature is increased or decreased by. Endothermic Reaction Decrease Temperature.
From improove.in
Experiment 16 Heat generation during reaction Improove Endothermic Reaction Decrease Temperature When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. Dissolution of ammonium nitrate in water, as in. This shifts. Endothermic Reaction Decrease Temperature.
From joizcduqw.blob.core.windows.net
Enzyme Chemical Meaning at Dexter Preston blog Endothermic Reaction Decrease Temperature Dissolution of ammonium nitrate in water, as in. This shifts chemical equilibria toward the products or. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. A temperature change occurs when temperature is increased or decreased by the flow of heat. In the course of an endothermic process, the system gains heat from the surroundings and so the. Endothermic Reaction Decrease Temperature.
From in.pinterest.com
Learn about exothermic and endothermic reactions in this blog post here Endothermic Reaction Decrease Temperature Dissolution of ammonium nitrate in water, as in. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. This shifts. Endothermic Reaction Decrease Temperature.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction Decrease Temperature The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become colder. A temperature change occurs when temperature is increased or decreased by the flow of heat. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets. This shifts chemical equilibria. Endothermic Reaction Decrease Temperature.
From www.slideserve.com
PPT Le Chatelier’s Principle PowerPoint Presentation, free download Endothermic Reaction Decrease Temperature By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. A thermometer is used to detect the temperature decrease. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. In the course of an endothermic process, the system gains heat from the surroundings and so. Endothermic Reaction Decrease Temperature.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Decrease Temperature A temperature change occurs when temperature is increased or decreased by the flow of heat. A thermometer is used to detect the temperature decrease. When energy is absorbed in an endothermic reaction, the temperature decreases. When energy is taken in from the surroundings, this is called an endothermic close endothermic reaction in which energy is taken in. The energy is. Endothermic Reaction Decrease Temperature.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Endothermic Reaction Decrease Temperature This shifts chemical equilibria toward the products or. A temperature change occurs when temperature is increased or decreased by the flow of heat. Dissolution of ammonium nitrate in water, as in. The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become colder. Thus, an endothermic reaction generally leads to an increase in the. Endothermic Reaction Decrease Temperature.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Decrease Temperature A temperature change occurs when temperature is increased or decreased by the flow of heat. When energy is taken in from the surroundings, this is called an endothermic close endothermic reaction in which energy is taken in. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Dissolution of ammonium nitrate in water, as in. This shifts chemical. Endothermic Reaction Decrease Temperature.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction Decrease Temperature When energy is absorbed in an endothermic reaction, the temperature decreases. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets. This shifts chemical equilibria toward the products or. A temperature change occurs when temperature is increased or decreased by the flow of heat. Thus, an endothermic. Endothermic Reaction Decrease Temperature.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Decrease Temperature A temperature change occurs when temperature is increased or decreased by the flow of heat. When energy is taken in from the surroundings, this is called an endothermic close endothermic reaction in which energy is taken in. The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become colder. Dissolution of ammonium nitrate in. Endothermic Reaction Decrease Temperature.
From circuitdblandlady.z21.web.core.windows.net
Energy Diagram For Exothermic Reaction Endothermic Reaction Decrease Temperature Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. When energy is absorbed in an endothermic reaction, the temperature decreases. A thermometer is used to detect the temperature decrease. A temperature change occurs when temperature is increased or decreased by the flow of heat. Dissolution of. Endothermic Reaction Decrease Temperature.
From www.reddit.com
Logic of Exothermic, Endothermic and Entropy So, Going from a gas to a Endothermic Reaction Decrease Temperature Dissolution of ammonium nitrate in water, as in. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. When energy is absorbed in an endothermic reaction, the temperature decreases. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. A thermometer is used to detect. Endothermic Reaction Decrease Temperature.
From schematicmandorlas.z14.web.core.windows.net
Energy Diagram For An Endothermic Reaction Endothermic Reaction Decrease Temperature A thermometer is used to detect the temperature decrease. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Dissolution of ammonium nitrate in water, as in. This shifts chemical equilibria toward the products or. A temperature change occurs when temperature is increased or decreased by the flow of heat. When energy is taken in from the surroundings,. Endothermic Reaction Decrease Temperature.
From en.ppt-online.org
Thermal Energy, Chemical Energy online presentation Endothermic Reaction Decrease Temperature When energy is absorbed in an endothermic reaction, the temperature decreases. The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become colder. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. Dissolution of ammonium nitrate in water, as in. A temperature change occurs when temperature is increased or decreased by. Endothermic Reaction Decrease Temperature.
From sciencenotes.org
Le Chatelier's Principle Endothermic Reaction Decrease Temperature This shifts chemical equilibria toward the products or. Dissolution of ammonium nitrate in water, as in. A temperature change occurs when temperature is increased or decreased by the flow of heat. When energy is absorbed in an endothermic reaction, the temperature decreases. The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become colder.. Endothermic Reaction Decrease Temperature.
From www.numerade.com
SOLVED Question 3 (4 points) Listen Under which conditions is a Endothermic Reaction Decrease Temperature A thermometer is used to detect the temperature decrease. The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become colder. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. When energy is taken in from the surroundings, this is called an endothermic close endothermic reaction in which energy is taken. Endothermic Reaction Decrease Temperature.
From loehrbtpf.blob.core.windows.net
Endothermic Reaction Thermal at John Maxey blog Endothermic Reaction Decrease Temperature When energy is absorbed in an endothermic reaction, the temperature decreases. The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become colder. Dissolution of ammonium nitrate in water, as in. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g. In the course of an endothermic process, the system gains heat. Endothermic Reaction Decrease Temperature.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Decrease Temperature The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become colder. A temperature change occurs when temperature is increased or decreased by the flow of heat. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. By definition, an exothermic reaction gives off heat, and endothermic reaction (e.g.. Endothermic Reaction Decrease Temperature.
From joifcnqsy.blob.core.windows.net
Does Partial Pressure Increase With Temperature at Linda Adams blog Endothermic Reaction Decrease Temperature Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. A thermometer is used to detect the temperature decrease. A temperature change occurs when temperature is increased or decreased by the flow of heat. When energy is absorbed in an endothermic reaction, the temperature decreases. The energy. Endothermic Reaction Decrease Temperature.
From khatiar1955a.altervista.org
The Difference Between Endothermic and Exothermic Reactions Endothermic Reaction Decrease Temperature When energy is absorbed in an endothermic reaction, the temperature decreases. This shifts chemical equilibria toward the products or. Dissolution of ammonium nitrate in water, as in. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets. When energy is taken in from the surroundings, this is. Endothermic Reaction Decrease Temperature.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Decrease Temperature When energy is absorbed in an endothermic reaction, the temperature decreases. A temperature change occurs when temperature is increased or decreased by the flow of heat. This shifts chemical equilibria toward the products or. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets. The energy is. Endothermic Reaction Decrease Temperature.
From musicbykatie.com
Does Temperature Increase Or Decrease Solubility In Endothermic Endothermic Reaction Decrease Temperature In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. A thermometer is used to detect the temperature decrease. When energy is taken in. Endothermic Reaction Decrease Temperature.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Decrease Temperature The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become colder. Dissolution of ammonium nitrate in water, as in. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. This shifts chemical equilibria toward the products or. In the course of an endothermic process, the system gains heat. Endothermic Reaction Decrease Temperature.
From www.numerade.com
SOLVED An endothermIc reaction causes the surroundings to Multlple Endothermic Reaction Decrease Temperature In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets. A thermometer is used to detect the temperature decrease. Dissolution of ammonium nitrate in water, as in. A temperature change occurs when temperature is increased or decreased by the flow of heat. When energy is taken in. Endothermic Reaction Decrease Temperature.
From www.expii.com
Spontaneous and Nonspontaneous Reactions — Overview Expii Endothermic Reaction Decrease Temperature In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. When energy is taken in from the surroundings, this is called an endothermic close. Endothermic Reaction Decrease Temperature.