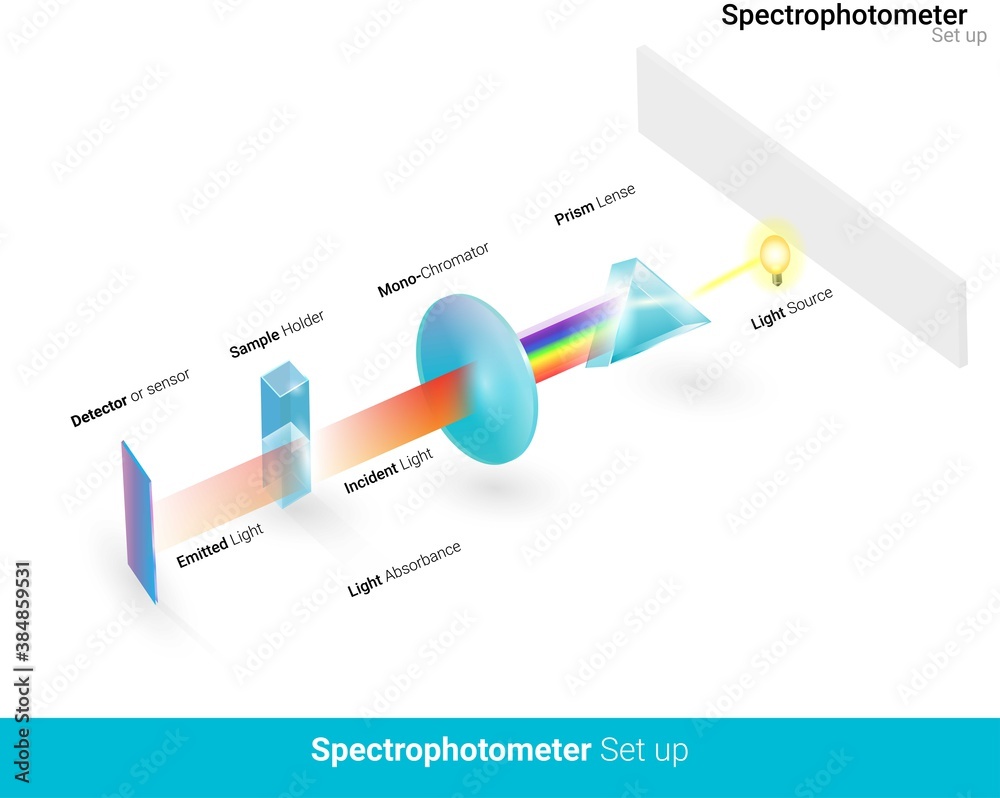
Spectrophotometry Beer Lambert Law . It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species.
from stock.adobe.com
This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species.
schematic diagram of spectrophotometer, UV visible spectrophotometer
Spectrophotometry Beer Lambert Law It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species.
From joibnsohl.blob.core.windows.net
Spectrophotometry Learn The BeerLambert Law With Absorbance Spectrophotometry Beer Lambert Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. This relationship is called. Spectrophotometry Beer Lambert Law.
From stock.adobe.com
schematic diagram of spectrophotometer, UV visible spectrophotometer Spectrophotometry Beer Lambert Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb. Spectrophotometry Beer Lambert Law.
From www.youtube.com
Spectrophotometric terms and BeerLambert Law YouTube Spectrophotometry Beer Lambert Law This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a). Spectrophotometry Beer Lambert Law.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Spectrophotometry Beer Lambert Law In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb. Spectrophotometry Beer Lambert Law.
From dxofafnaw.blob.core.windows.net
Beer Lambert Law Definition Chemistry at Myrtle blog Spectrophotometry Beer Lambert Law It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a). Spectrophotometry Beer Lambert Law.
From www.youtube.com
Introduction to UVVis Spectroscopy 03 BeerLambert Law YouTube Spectrophotometry Beer Lambert Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a). Spectrophotometry Beer Lambert Law.
From byjus.com
State and explain Beer Lambert Law. Spectrophotometry Beer Lambert Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called the. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb. Spectrophotometry Beer Lambert Law.
From studylib.net
Beer Lambert`s law, Raman spectroscopy Spectrophotometry Beer Lambert Law In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called. Spectrophotometry Beer Lambert Law.
From slidetodoc.com
Part 2 9 Electronic Transitions Outline Absorption spectroscopy Spectrophotometry Beer Lambert Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. This relationship is called. Spectrophotometry Beer Lambert Law.
From www.youtube.com
Beer's Law Overview YouTube Spectrophotometry Beer Lambert Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called. Spectrophotometry Beer Lambert Law.
From mungfali.com
UV Spectroscopy Beer Lambert Law Spectrophotometry Beer Lambert Law In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb. Spectrophotometry Beer Lambert Law.
From study.com
BeerLambert Law Equation, Units & Examples Lesson Spectrophotometry Beer Lambert Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a). Spectrophotometry Beer Lambert Law.
From joimeihka.blob.core.windows.net
Beer Lambert Law Linear Equation at Kimberly Graves blog Spectrophotometry Beer Lambert Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. This relationship is called. Spectrophotometry Beer Lambert Law.
From www.researchgate.net
1 BeerLambert law; Xrays to solid matter interaction. Download Spectrophotometry Beer Lambert Law In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called. Spectrophotometry Beer Lambert Law.
From www.youtube.com
Absorption spectroscopy (BeerLambert law) presented by PhD Emil Spectrophotometry Beer Lambert Law This relationship is called the. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. Spectrophotometry Beer Lambert Law.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Spectrophotometry Beer Lambert Law This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb. Spectrophotometry Beer Lambert Law.
From exorduurt.blob.core.windows.net
Why Beer Lambert Law Fails At Higher Concentrations at Rose Narvaez blog Spectrophotometry Beer Lambert Law In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb. Spectrophotometry Beer Lambert Law.
From edu.rsc.org
Smartphone spectroscopy BeerLambert law Resource RSC Education Spectrophotometry Beer Lambert Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. This relationship is called. Spectrophotometry Beer Lambert Law.
From www.youtube.com
Spectroscopy Beer Lambert's Law YouTube Spectrophotometry Beer Lambert Law It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a). Spectrophotometry Beer Lambert Law.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Spectrophotometry Beer Lambert Law This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb. Spectrophotometry Beer Lambert Law.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Spectrophotometry Beer Lambert Law This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb. Spectrophotometry Beer Lambert Law.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Spectrophotometry Beer Lambert Law It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. Spectrophotometry Beer Lambert Law.
From dxofafnaw.blob.core.windows.net
Beer Lambert Law Definition Chemistry at Myrtle blog Spectrophotometry Beer Lambert Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb. Spectrophotometry Beer Lambert Law.
From www.youtube.com
Spectrophotometry BeerLambert Law. YouTube Spectrophotometry Beer Lambert Law In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called the. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. Spectrophotometry Beer Lambert Law.
From derangedphysiology.com
Absorption spectroscopy of haemoglobin species Deranged Physiology Spectrophotometry Beer Lambert Law It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a). Spectrophotometry Beer Lambert Law.
From www.slideserve.com
PPT Absorption Spectroscopy of Biopolymers PowerPoint Presentation Spectrophotometry Beer Lambert Law In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called the. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. Spectrophotometry Beer Lambert Law.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Spectrophotometry Beer Lambert Law It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. Spectrophotometry Beer Lambert Law.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Spectrophotometry Beer Lambert Law This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. Spectrophotometry Beer Lambert Law.
From www.thoughtco.com
Beer's Law Definition and Equation Spectrophotometry Beer Lambert Law In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb. Spectrophotometry Beer Lambert Law.
From klaorzemv.blob.core.windows.net
Beer Lambert Law Constant at Rebecca Curtis blog Spectrophotometry Beer Lambert Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a). Spectrophotometry Beer Lambert Law.
From hudsontinhoffman.blogspot.com
Beer's Lambert Law Equation HudsontinHoffman Spectrophotometry Beer Lambert Law This relationship is called the. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing. Spectrophotometry Beer Lambert Law.
From studylib.net
Casestudy The BeerLambert Law and Spectrophotometry Learning objectives Spectrophotometry Beer Lambert Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb. Spectrophotometry Beer Lambert Law.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Spectrophotometry Beer Lambert Law In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb. Spectrophotometry Beer Lambert Law.
From mungfali.com
UV Spectroscopy Beer Lambert Law Spectrophotometry Beer Lambert Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. This relationship is called. Spectrophotometry Beer Lambert Law.
From www.slideserve.com
PPT Chemical the rates of reactions PowerPoint Presentation Spectrophotometry Beer Lambert Law The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called. Spectrophotometry Beer Lambert Law.