Calorimetry And Specific Heat Lab Report Conclusion . Design the calorimetry constant experiment ;. Heat is a form of energy that is transferred between objects with different temperatures. page 1 of 4. Chemistry document from poughkeepsie high school, 4 pages, kiara scott september 17 2020. in conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. summary you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. anna mitchell chemistry 11/10/ calorimetry and specific heat lab report the purpose of this lab was to explore how the specific. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to a maximum of. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic.
from www.studocu.com
anna mitchell chemistry 11/10/ calorimetry and specific heat lab report the purpose of this lab was to explore how the specific. summary you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. Heat is a form of energy that is transferred between objects with different temperatures. Chemistry document from poughkeepsie high school, 4 pages, kiara scott september 17 2020. Design the calorimetry constant experiment ;. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to a maximum of. in conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. page 1 of 4.
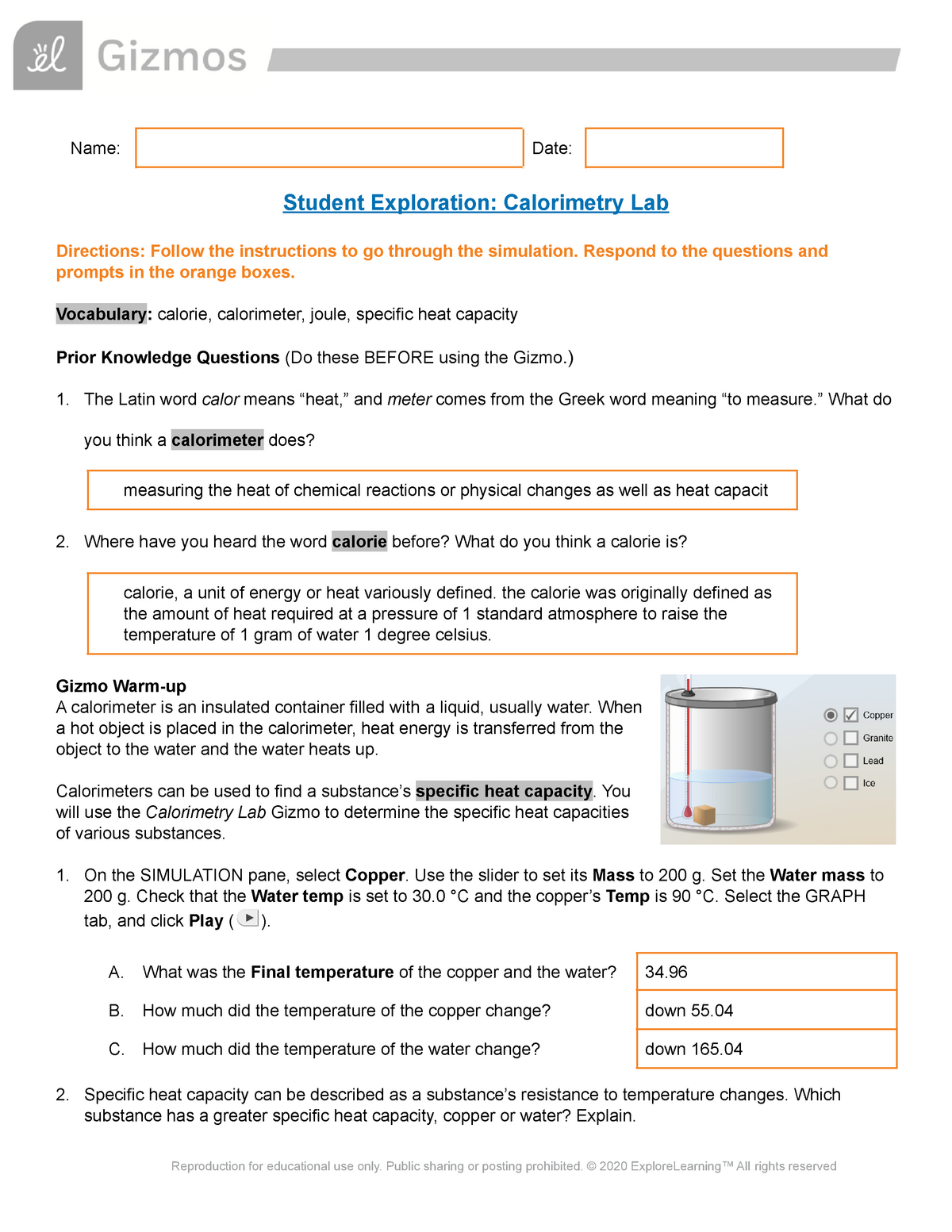
Calorimetry Gizmo Lab Name Date Student Exploration Calorimetry
Calorimetry And Specific Heat Lab Report Conclusion conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. page 1 of 4. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to a maximum of. Heat is a form of energy that is transferred between objects with different temperatures. Chemistry document from poughkeepsie high school, 4 pages, kiara scott september 17 2020. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. anna mitchell chemistry 11/10/ calorimetry and specific heat lab report the purpose of this lab was to explore how the specific. in conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. summary you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. Design the calorimetry constant experiment ;.
From brainly.com
Does anyone have the table for the Calorimetry and Specific Heat lab Calorimetry And Specific Heat Lab Report Conclusion Heat is a form of energy that is transferred between objects with different temperatures. summary you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. page 1 of 4. Chemistry document from poughkeepsie high school, 4 pages, kiara scott september 17 2020. when 1.104 grams of iron. Calorimetry And Specific Heat Lab Report Conclusion.
From www.studocu.com
Heat Effects and Calorimetry Lab Report Name Akzhan Yestoreyeva Date Calorimetry And Specific Heat Lab Report Conclusion page 1 of 4. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to a maximum of. Heat is a form of energy that is transferred between objects with different temperatures. anna mitchell chemistry 11/10/ calorimetry and specific heat lab report. Calorimetry And Specific Heat Lab Report Conclusion.
From www.studocu.com
Calorimetry Gizmo Lab Name Date Student Exploration Calorimetry Calorimetry And Specific Heat Lab Report Conclusion Design the calorimetry constant experiment ;. anna mitchell chemistry 11/10/ calorimetry and specific heat lab report the purpose of this lab was to explore how the specific. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. when 1.104 grams of iron metal are mixed with 26.023 grams. Calorimetry And Specific Heat Lab Report Conclusion.
From www.studypool.com
SOLUTION Lab report calorimetry and specific heat Studypool Calorimetry And Specific Heat Lab Report Conclusion conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. Heat is a form of energy that is transferred between objects with different temperatures. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c. Calorimetry And Specific Heat Lab Report Conclusion.
From www.studypool.com
SOLUTION Lab calorimetry and specific heat test 2023 Studypool Calorimetry And Specific Heat Lab Report Conclusion page 1 of 4. Heat is a form of energy that is transferred between objects with different temperatures. Chemistry document from poughkeepsie high school, 4 pages, kiara scott september 17 2020. in conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. conclusion in conclusion, the results for the average heat capacity. Calorimetry And Specific Heat Lab Report Conclusion.
From grade12uchem.weebly.com
Calorimetry latest copy of grade 12 U Calorimetry And Specific Heat Lab Report Conclusion conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. Chemistry document from poughkeepsie high school, 4 pages, kiara scott september 17 2020. page 1 of 4. Heat is a form of energy that is transferred between objects with different temperatures. anna mitchell chemistry 11/10/ calorimetry and specific. Calorimetry And Specific Heat Lab Report Conclusion.
From www.scribd.com
Calorimetry Lab SE PDF Calorie Heat Capacity Calorimetry And Specific Heat Lab Report Conclusion Heat is a form of energy that is transferred between objects with different temperatures. Design the calorimetry constant experiment ;. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. summary you will use a calorimeter to collect data that enables you to calculate the specific heat of all. Calorimetry And Specific Heat Lab Report Conclusion.
From www.chegg.com
1/3 > Calorimetry and Specific Heat Lab You work for Calorimetry And Specific Heat Lab Report Conclusion summary you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. in conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. anna mitchell chemistry 11/10/ calorimetry and specific heat lab report the purpose of this lab was to explore how the. Calorimetry And Specific Heat Lab Report Conclusion.
From www.chegg.com
1/3 > Calorimetry and Specific Heat Lab You work for Calorimetry And Specific Heat Lab Report Conclusion Heat is a form of energy that is transferred between objects with different temperatures. in conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. page 1 of 4. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. when 1.104 grams. Calorimetry And Specific Heat Lab Report Conclusion.
From www.chegg.com
Lab 3. Calorimetry Measurement of Specific Heat Calorimetry And Specific Heat Lab Report Conclusion Chemistry document from poughkeepsie high school, 4 pages, kiara scott september 17 2020. page 1 of 4. anna mitchell chemistry 11/10/ calorimetry and specific heat lab report the purpose of this lab was to explore how the specific. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic.. Calorimetry And Specific Heat Lab Report Conclusion.
From www.chegg.com
Section Energy Changes, Calorimetry and Specific Heat Calorimetry And Specific Heat Lab Report Conclusion conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. Heat is a form of energy that is transferred between objects with different temperatures. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c. Calorimetry And Specific Heat Lab Report Conclusion.
From answerhappy.com
CALORIMETRY HEAT CAPACITY OF A CALORIMETER NTRODUCTION Lab Data Calorimetry And Specific Heat Lab Report Conclusion summary you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to a maximum of. Design the calorimetry constant experiment ;. Heat is. Calorimetry And Specific Heat Lab Report Conclusion.
From users.highland.edu
Calorimetry Calorimetry And Specific Heat Lab Report Conclusion when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to a maximum of. summary you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. Heat is a form of energy that is. Calorimetry And Specific Heat Lab Report Conclusion.
From www.scribd.com
Lab 07Specific Heat & Calorimetry.pdf Heat Temperature Calorimetry And Specific Heat Lab Report Conclusion page 1 of 4. Chemistry document from poughkeepsie high school, 4 pages, kiara scott september 17 2020. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. anna mitchell chemistry 11/10/ calorimetry and specific heat lab report the purpose of this lab was to explore how the specific.. Calorimetry And Specific Heat Lab Report Conclusion.
From rainis.pics
Calorimeter Types and Heat Flow Analysis (M6Q5) UWMadison Chemistry Calorimetry And Specific Heat Lab Report Conclusion when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to a maximum of. summary you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. conclusion in conclusion, the results for the. Calorimetry And Specific Heat Lab Report Conclusion.
From www.studocu.com
Calorimetry Lab Report Name Mahma Ahmed Partner Wala Naeem Student Calorimetry And Specific Heat Lab Report Conclusion Heat is a form of energy that is transferred between objects with different temperatures. anna mitchell chemistry 11/10/ calorimetry and specific heat lab report the purpose of this lab was to explore how the specific. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from. Calorimetry And Specific Heat Lab Report Conclusion.
From www.studypool.com
SOLUTION Lab calorimetry and specific heat test 2023 Studypool Calorimetry And Specific Heat Lab Report Conclusion Design the calorimetry constant experiment ;. Heat is a form of energy that is transferred between objects with different temperatures. anna mitchell chemistry 11/10/ calorimetry and specific heat lab report the purpose of this lab was to explore how the specific. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect. Calorimetry And Specific Heat Lab Report Conclusion.
From brainly.com
calorimetry and specific heat lab report can someone please write this Calorimetry And Specific Heat Lab Report Conclusion summary you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. in conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the. Calorimetry And Specific Heat Lab Report Conclusion.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimetry And Specific Heat Lab Report Conclusion Design the calorimetry constant experiment ;. Heat is a form of energy that is transferred between objects with different temperatures. in conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. summary you. Calorimetry And Specific Heat Lab Report Conclusion.
From studylib.net
06.03 Calorimetry Lab Report Calorimetry And Specific Heat Lab Report Conclusion summary you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. page 1 of 4. in conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. conclusion in conclusion, the results for the average heat capacity for all of these procedures. Calorimetry And Specific Heat Lab Report Conclusion.
From mmsphyschem.com
Calorimetry MM's site Calorimetry And Specific Heat Lab Report Conclusion Design the calorimetry constant experiment ;. Chemistry document from poughkeepsie high school, 4 pages, kiara scott september 17 2020. Heat is a form of energy that is transferred between objects with different temperatures. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to. Calorimetry And Specific Heat Lab Report Conclusion.
From www.baamboozle.com
Heat Gain and Heat Loss Baamboozle Baamboozle The Most Fun Calorimetry And Specific Heat Lab Report Conclusion in conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. anna mitchell chemistry 11/10/ calorimetry and specific heat lab report the purpose of this lab was to explore how the specific. summary you will use a calorimeter to collect data that enables you to calculate the specific heat of all four. Calorimetry And Specific Heat Lab Report Conclusion.
From www.chegg.com
Solved Calorimetry Lab Report o PART A. Specific Heat Calorimetry And Specific Heat Lab Report Conclusion page 1 of 4. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. summary you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. Chemistry document from poughkeepsie high school, 4 pages, kiara scott september 17 2020.. Calorimetry And Specific Heat Lab Report Conclusion.
From www.studypool.com
SOLUTION Gizmo student exploration calorimetry lab answer key Studypool Calorimetry And Specific Heat Lab Report Conclusion in conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. Chemistry document from poughkeepsie high school, 4 pages, kiara scott september 17 2020. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to a maximum of.. Calorimetry And Specific Heat Lab Report Conclusion.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimetry And Specific Heat Lab Report Conclusion when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to a maximum of. page 1 of 4. in conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. Heat is a form of energy that is. Calorimetry And Specific Heat Lab Report Conclusion.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry And Specific Heat Lab Report Conclusion in conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to a maximum of. Heat is a form of energy that is transferred between objects with different. Calorimetry And Specific Heat Lab Report Conclusion.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimetry And Specific Heat Lab Report Conclusion when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to a maximum of. page 1 of 4. in conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. anna mitchell chemistry 11/10/ calorimetry and specific. Calorimetry And Specific Heat Lab Report Conclusion.
From www.studocu.com
Calorimetry (Experiment 3) Laboratory Report Calorimetry (Experiment Calorimetry And Specific Heat Lab Report Conclusion Heat is a form of energy that is transferred between objects with different temperatures. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to a maximum of. anna mitchell chemistry 11/10/ calorimetry and specific heat lab report the purpose of this lab. Calorimetry And Specific Heat Lab Report Conclusion.
From www.chegg.com
Solved REPORT FOR EXPERIMENT5 Calorimetry and Specific Heat Calorimetry And Specific Heat Lab Report Conclusion Design the calorimetry constant experiment ;. Chemistry document from poughkeepsie high school, 4 pages, kiara scott september 17 2020. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to a maximum of. page 1 of 4. Heat is a form of energy. Calorimetry And Specific Heat Lab Report Conclusion.
From www.pinterest.com
Calorimetry Lab Report in 2022 Science diagrams, Lab report Calorimetry And Specific Heat Lab Report Conclusion conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. Chemistry document from poughkeepsie high school, 4 pages, kiara scott september 17 2020. page 1 of 4. Design the calorimetry constant experiment ;. summary you will use a calorimeter to collect data that enables you to calculate the. Calorimetry And Specific Heat Lab Report Conclusion.
From www.studocu.com
P1 Lab Calorimetry and Specific Heat Lab Calorimetry and Specific Calorimetry And Specific Heat Lab Report Conclusion Design the calorimetry constant experiment ;. Chemistry document from poughkeepsie high school, 4 pages, kiara scott september 17 2020. Heat is a form of energy that is transferred between objects with different temperatures. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to. Calorimetry And Specific Heat Lab Report Conclusion.
From mnriverpokercom.blogspot.com
Calorimetry Lab Gizmo Answers Activity C Gizmo Conduction And Calorimetry And Specific Heat Lab Report Conclusion Heat is a form of energy that is transferred between objects with different temperatures. Design the calorimetry constant experiment ;. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to a maximum of. anna mitchell chemistry 11/10/ calorimetry and specific heat lab. Calorimetry And Specific Heat Lab Report Conclusion.
From www.chegg.com
1/3 > Calorimetry and Specific Heat Lab You work for Calorimetry And Specific Heat Lab Report Conclusion Heat is a form of energy that is transferred between objects with different temperatures. anna mitchell chemistry 11/10/ calorimetry and specific heat lab report the purpose of this lab was to explore how the specific. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. in conclusion, the. Calorimetry And Specific Heat Lab Report Conclusion.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry And Specific Heat Lab Report Conclusion Chemistry document from poughkeepsie high school, 4 pages, kiara scott september 17 2020. conclusion in conclusion, the results for the average heat capacity for all of these procedures should reflect the endothermic. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to. Calorimetry And Specific Heat Lab Report Conclusion.
From www.chegg.com
Solved Calorimetry Lab Report Eisa Khalid Name 600 mwa how Calorimetry And Specific Heat Lab Report Conclusion page 1 of 4. when 1.104 grams of iron metal are mixed with 26.023 grams of hydrochloric acid in a coffee cup calorimeter, the temperature rises from 25.2 °c to a maximum of. in conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. Chemistry document from poughkeepsie high school, 4 pages,. Calorimetry And Specific Heat Lab Report Conclusion.