Catalytic Meaning With Example . Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. Learn what a catalyst is and how it affects the rate and path of chemical reactions. Learn about heterogeneous and homogeneous catalysis, and how they work. Learn about the different types of catalysts, how they work, and some examples of. Find out the types of catalytic reactions,. Learn more about catalytic processes, such as oxidation, cracking, and. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. See examples of catalytic reactions, such as hydrogenation, and autocatalysis. Explore the different types of catalysts, such as positive, negative,. Catalytic means using a catalyst to speed up a chemical reaction. Learn about catalysis, the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a substance that increases the rate of a chemical reaction without being consumed.
from ppt-online.org
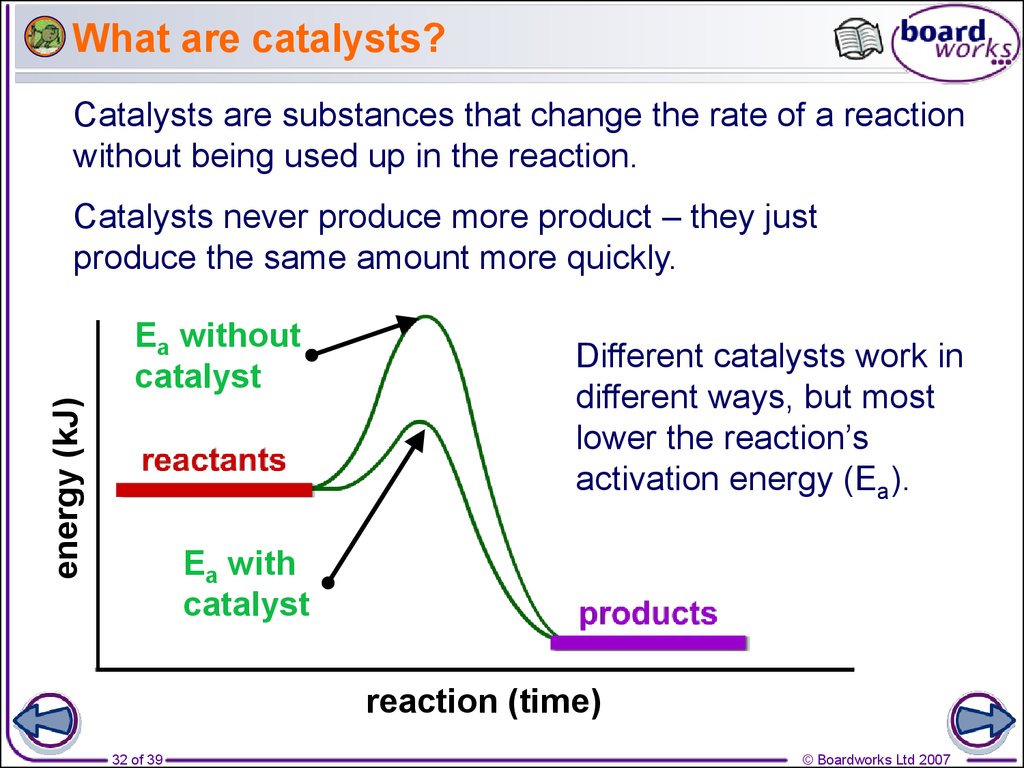
A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Explore the different types of catalysts, such as positive, negative,. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. Learn about catalysis, the process that alters the rate of a chemical reaction under the influence of a catalyst. Learn what a catalyst is and how it affects the rate and path of chemical reactions. Find out the types of catalytic reactions,. Learn about heterogeneous and homogeneous catalysis, and how they work. Catalytic means using a catalyst to speed up a chemical reaction. Learn more about catalytic processes, such as oxidation, cracking, and. See examples of catalytic reactions, such as hydrogenation, and autocatalysis.
Rates of reaction презентация онлайн
Catalytic Meaning With Example Learn about heterogeneous and homogeneous catalysis, and how they work. Catalytic means using a catalyst to speed up a chemical reaction. A catalyst is a substance that increases the rate of a chemical reaction without being consumed. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn about catalysis, the process that alters the rate of a chemical reaction under the influence of a catalyst. See examples of catalytic reactions, such as hydrogenation, and autocatalysis. Learn about heterogeneous and homogeneous catalysis, and how they work. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. Explore the different types of catalysts, such as positive, negative,. Learn more about catalytic processes, such as oxidation, cracking, and. Learn what a catalyst is and how it affects the rate and path of chemical reactions. Learn about the different types of catalysts, how they work, and some examples of. Find out the types of catalytic reactions,.
From brainly.in
define catalyst with example ?? Brainly.in Catalytic Meaning With Example Learn more about catalytic processes, such as oxidation, cracking, and. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. Catalytic means using a catalyst to speed up a chemical reaction. Explore the different types of. Catalytic Meaning With Example.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalytic Meaning With Example Learn about catalysis, the process that alters the rate of a chemical reaction under the influence of a catalyst. Learn more about catalytic processes, such as oxidation, cracking, and. Find out the types of catalytic reactions,. Learn about heterogeneous and homogeneous catalysis, and how they work. Explore the different types of catalysts, such as positive, negative,. Learn about different types. Catalytic Meaning With Example.
From www.researchgate.net
Catalytic Reforming Flowchart (SemiRegenerative) Download Scientific Catalytic Meaning With Example A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Find out the types of catalytic reactions,. Learn about the different types of catalysts, how they work, and some examples of. See examples of catalytic reactions, such as hydrogenation, and autocatalysis. Catalytic means using a catalyst to speed up a chemical reaction.. Catalytic Meaning With Example.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalytic Meaning With Example Learn more about catalytic processes, such as oxidation, cracking, and. Learn about the different types of catalysts, how they work, and some examples of. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. A catalyst is a substance that increases the rate of a chemical reaction without being consumed. Learn about catalysis, the. Catalytic Meaning With Example.
From www.differencebetween.com
Difference Between Catalytic and Non Catalytic Reaction Compare the Catalytic Meaning With Example Learn about the different types of catalysts, how they work, and some examples of. Find out the types of catalytic reactions,. Learn more about catalytic processes, such as oxidation, cracking, and. A catalyst is a substance that increases the rate of a chemical reaction without being consumed. Learn about catalysis, the process that alters the rate of a chemical reaction. Catalytic Meaning With Example.
From www.worksheetsplanet.com
What is a Catalyst Definition of Catalyst Catalytic Meaning With Example Find out the types of catalytic reactions,. Learn about the different types of catalysts, how they work, and some examples of. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. Learn more about catalytic processes, such as oxidation, cracking, and. See examples of catalytic reactions, such as hydrogenation, and autocatalysis. Catalytic means using. Catalytic Meaning With Example.
From www.youtube.com
Catalytic activity Meaning YouTube Catalytic Meaning With Example Find out the types of catalytic reactions,. Explore the different types of catalysts, such as positive, negative,. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. Catalytic means using a catalyst to speed up a chemical reaction. Learn about the different types of catalysts, how they work, and some examples of. A catalyst. Catalytic Meaning With Example.
From joisnvcab.blob.core.windows.net
Catalyst Meaning Collins at Karl Luc blog Catalytic Meaning With Example Learn what a catalyst is and how it affects the rate and path of chemical reactions. Learn about catalysis, the process that alters the rate of a chemical reaction under the influence of a catalyst. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. Learn about the different types of catalysts, how they. Catalytic Meaning With Example.
From www.slideserve.com
PPT Catalysis PowerPoint Presentation, free download ID2824547 Catalytic Meaning With Example A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn what a catalyst is and how it affects the rate and path of chemical reactions. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. Explore the different types of catalysts, such as positive, negative,.. Catalytic Meaning With Example.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalytic Meaning With Example Learn about the different types of catalysts, how they work, and some examples of. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. Learn more about catalytic processes, such as oxidation, cracking, and. Catalytic means using a catalyst to speed up a chemical reaction. A catalyst is a substance that increases the rate. Catalytic Meaning With Example.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalytic Meaning With Example Learn what a catalyst is and how it affects the rate and path of chemical reactions. Catalytic means using a catalyst to speed up a chemical reaction. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. See examples of catalytic reactions, such as hydrogenation, and autocatalysis. Learn about heterogeneous and homogeneous. Catalytic Meaning With Example.
From joicdqxxo.blob.core.windows.net
Catalyst Development Definition at Ruth Cruz blog Catalytic Meaning With Example See examples of catalytic reactions, such as hydrogenation, and autocatalysis. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. Learn more about catalytic processes, such as oxidation, cracking, and. Learn about heterogeneous and homogeneous catalysis, and how they work. Catalytic means using a catalyst to speed up a chemical reaction. Explore the different. Catalytic Meaning With Example.
From joilxpqmt.blob.core.windows.net
Chemical Catalysis Examples at Howard Wade blog Catalytic Meaning With Example Learn about the different types of catalysts, how they work, and some examples of. Explore the different types of catalysts, such as positive, negative,. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. Learn about heterogeneous and homogeneous catalysis, and how they work. Find out the types of catalytic reactions,. See examples of. Catalytic Meaning With Example.
From exoieyaef.blob.core.windows.net
Catalyst Def Biology at Ethel Hughes blog Catalytic Meaning With Example Learn about heterogeneous and homogeneous catalysis, and how they work. Learn more about catalytic processes, such as oxidation, cracking, and. Find out the types of catalytic reactions,. Catalytic means using a catalyst to speed up a chemical reaction. A catalyst is a substance that increases the rate of a chemical reaction without being consumed. Learn about catalysis, the process that. Catalytic Meaning With Example.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalytic Meaning With Example See examples of catalytic reactions, such as hydrogenation, and autocatalysis. Learn about heterogeneous and homogeneous catalysis, and how they work. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn more about catalytic processes, such as oxidation, cracking, and. Learn what a catalyst is and how it affects the rate and. Catalytic Meaning With Example.
From www.slideshare.net
Catalyst Catalytic Meaning With Example Learn more about catalytic processes, such as oxidation, cracking, and. See examples of catalytic reactions, such as hydrogenation, and autocatalysis. A catalyst is a substance that increases the rate of a chemical reaction without being consumed. Learn about the different types of catalysts, how they work, and some examples of. Explore the different types of catalysts, such as positive, negative,.. Catalytic Meaning With Example.
From joifdozvf.blob.core.windows.net
What Is A Catalyst In Chemistry Example at Herbert Simpson blog Catalytic Meaning With Example Catalytic means using a catalyst to speed up a chemical reaction. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. See examples of catalytic reactions, such as hydrogenation, and autocatalysis. Explore the different types of catalysts, such as positive, negative,. A catalyst is a substance that increases the rate of a chemical reaction. Catalytic Meaning With Example.
From testbook.com
Catalysis in Chemistry Meaning , Catalyst Types & Examples. Catalytic Meaning With Example Learn about catalysis, the process that alters the rate of a chemical reaction under the influence of a catalyst. Learn about heterogeneous and homogeneous catalysis, and how they work. Learn more about catalytic processes, such as oxidation, cracking, and. Find out the types of catalytic reactions,. A catalyst is a substance that increases the rate of a chemical reaction without. Catalytic Meaning With Example.
From klaesyagl.blob.core.windows.net
Catalyst Meaning With Pronunciation at Amy Robinson blog Catalytic Meaning With Example Catalytic means using a catalyst to speed up a chemical reaction. Learn about the different types of catalysts, how they work, and some examples of. Learn about catalysis, the process that alters the rate of a chemical reaction under the influence of a catalyst. Explore the different types of catalysts, such as positive, negative,. Learn about heterogeneous and homogeneous catalysis,. Catalytic Meaning With Example.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2380033 Catalytic Meaning With Example Explore the different types of catalysts, such as positive, negative,. Learn about the different types of catalysts, how they work, and some examples of. Find out the types of catalytic reactions,. Learn more about catalytic processes, such as oxidation, cracking, and. Learn about catalysis, the process that alters the rate of a chemical reaction under the influence of a catalyst.. Catalytic Meaning With Example.
From chemistnotes.com
Acid base catalysis General vs specific Chemistry Notes Catalytic Meaning With Example Learn about the different types of catalysts, how they work, and some examples of. Learn more about catalytic processes, such as oxidation, cracking, and. A catalyst is a substance that increases the rate of a chemical reaction without being consumed. Learn about catalysis, the process that alters the rate of a chemical reaction under the influence of a catalyst. Learn. Catalytic Meaning With Example.
From klaesyagl.blob.core.windows.net
Catalyst Meaning With Pronunciation at Amy Robinson blog Catalytic Meaning With Example Explore the different types of catalysts, such as positive, negative,. See examples of catalytic reactions, such as hydrogenation, and autocatalysis. Find out the types of catalytic reactions,. Learn about heterogeneous and homogeneous catalysis, and how they work. A catalyst is a substance that increases the rate of a chemical reaction without being consumed. Learn about catalysis, the process that alters. Catalytic Meaning With Example.
From www.e-education.psu.edu
Fluid Catalytic Cracking (FCC) FSC 432 Petroleum Refining Catalytic Meaning With Example Catalytic means using a catalyst to speed up a chemical reaction. See examples of catalytic reactions, such as hydrogenation, and autocatalysis. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. Explore the different types of catalysts, such as positive, negative,. Learn more about catalytic processes, such as oxidation, cracking, and. Learn about heterogeneous. Catalytic Meaning With Example.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID3996834 Catalytic Meaning With Example Learn about catalysis, the process that alters the rate of a chemical reaction under the influence of a catalyst. Learn about the different types of catalysts, how they work, and some examples of. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. Explore the different types of catalysts, such as positive, negative,. Learn. Catalytic Meaning With Example.
From www.pinterest.com
Meaning of catalyst Reasoning Test, English Language Learning, Chemical Catalytic Meaning With Example Learn about the different types of catalysts, how they work, and some examples of. Find out the types of catalytic reactions,. Learn about heterogeneous and homogeneous catalysis, and how they work. Learn about catalysis, the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a substance that increases the rate of. Catalytic Meaning With Example.
From wordstodescribesomeone.com
catalyst definition catalyst meaning words to describe someone Catalytic Meaning With Example Learn about heterogeneous and homogeneous catalysis, and how they work. Explore the different types of catalysts, such as positive, negative,. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. Learn more about catalytic processes, such as oxidation, cracking, and. Learn about the different types of catalysts, how they work, and some examples of.. Catalytic Meaning With Example.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalytic Meaning With Example A catalyst is a substance that increases the rate of a chemical reaction without being consumed. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Explore the different types of catalysts, such as positive, negative,. Learn more about catalytic processes, such as oxidation, cracking, and. Learn what a catalyst is and. Catalytic Meaning With Example.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalytic Meaning With Example Learn about heterogeneous and homogeneous catalysis, and how they work. Learn about the different types of catalysts, how they work, and some examples of. Find out the types of catalytic reactions,. Learn about catalysis, the process that alters the rate of a chemical reaction under the influence of a catalyst. Learn what a catalyst is and how it affects the. Catalytic Meaning With Example.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalytic Meaning With Example See examples of catalytic reactions, such as hydrogenation, and autocatalysis. Explore the different types of catalysts, such as positive, negative,. Learn more about catalytic processes, such as oxidation, cracking, and. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. Learn what a catalyst is and how it affects the rate and path of. Catalytic Meaning With Example.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2823635 Catalytic Meaning With Example Explore the different types of catalysts, such as positive, negative,. Find out the types of catalytic reactions,. Learn more about catalytic processes, such as oxidation, cracking, and. Learn about heterogeneous and homogeneous catalysis, and how they work. See examples of catalytic reactions, such as hydrogenation, and autocatalysis. A catalyst is a substance that increases the rate of a chemical reaction. Catalytic Meaning With Example.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalytic Meaning With Example Learn what a catalyst is and how it affects the rate and path of chemical reactions. A catalyst is a substance that increases the rate of a chemical reaction without being consumed. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn about different types of catalysts, such as enzymes, metals,. Catalytic Meaning With Example.
From www.youtube.com
Catalytic Meaning YouTube Catalytic Meaning With Example Learn about catalysis, the process that alters the rate of a chemical reaction under the influence of a catalyst. Catalytic means using a catalyst to speed up a chemical reaction. Learn about heterogeneous and homogeneous catalysis, and how they work. Learn about the different types of catalysts, how they work, and some examples of. A catalyst is a substance that. Catalytic Meaning With Example.
From www.mdpi.com
Catalysts Free FullText FCC Catalyst Accessibility—A Review Catalytic Meaning With Example A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn about different types of catalysts, such as enzymes, metals, and zeolites, and how they work. See examples of catalytic reactions, such as hydrogenation, and autocatalysis. Catalytic means using a catalyst to speed up a chemical reaction. Learn about catalysis, the process. Catalytic Meaning With Example.
From ppt-online.org
Rates of reaction презентация онлайн Catalytic Meaning With Example Explore the different types of catalysts, such as positive, negative,. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn more about catalytic processes, such as oxidation, cracking, and. Learn about catalysis, the process that alters the rate of a chemical reaction under the influence of a catalyst. Catalytic means using. Catalytic Meaning With Example.
From meaningkosh.com
Catalytic Meaning In Hindi MeaningKosh Catalytic Meaning With Example Catalytic means using a catalyst to speed up a chemical reaction. Find out the types of catalytic reactions,. Learn what a catalyst is and how it affects the rate and path of chemical reactions. Learn about catalysis, the process that alters the rate of a chemical reaction under the influence of a catalyst. Learn about the different types of catalysts,. Catalytic Meaning With Example.