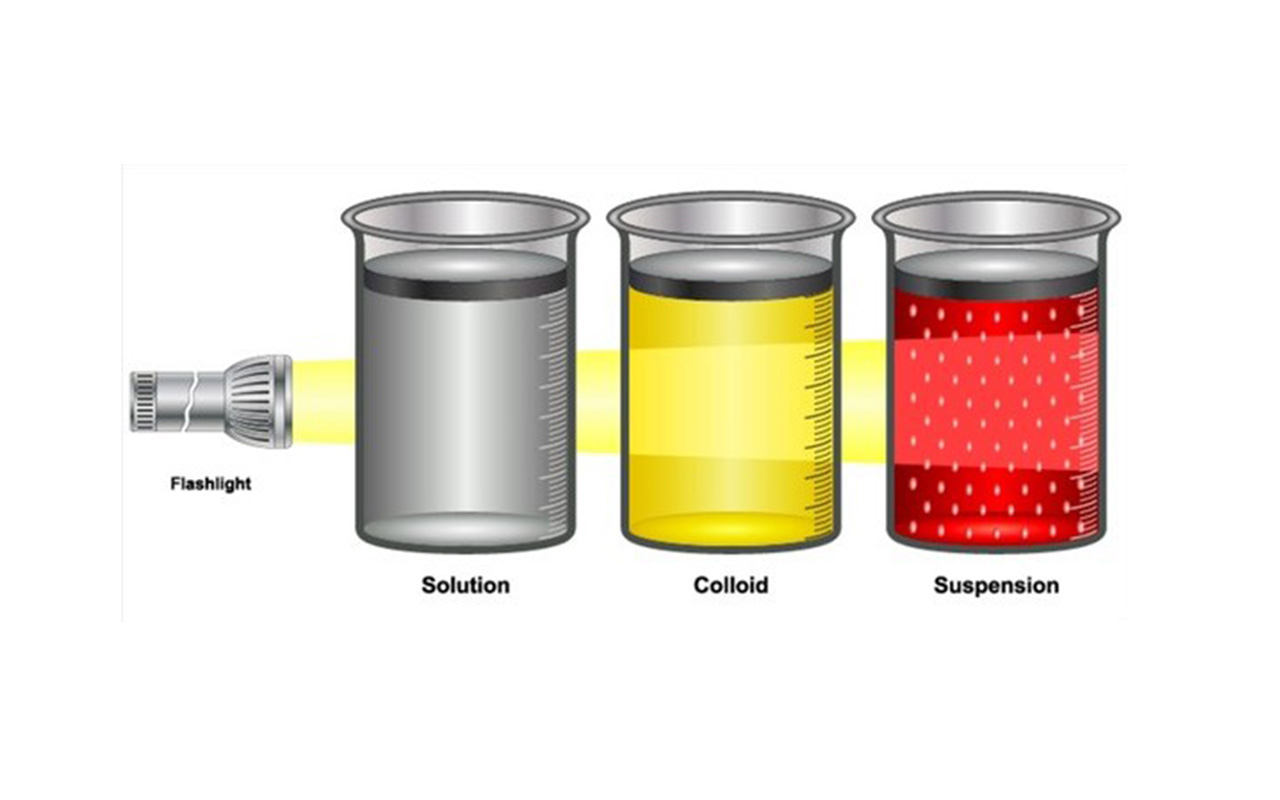
Colloid Solution Particle Size . Particles this small can neither be seen by the. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In solutions, particles range in size from 0.01 to one nanometer. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 11.6.1. Unlike true solutions where solute particles are dissolved at. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. In contrast, particles in a solution are smaller than this size, while particles in a. These particles can be solid, liquid, or gas. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension.
from joislngxm.blob.core.windows.net
A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 11.6.1. In contrast, particles in a solution are smaller than this size, while particles in a. These particles can be solid, liquid, or gas. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Particles this small can neither be seen by the. In solutions, particles range in size from 0.01 to one nanometer. Unlike true solutions where solute particles are dissolved at. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter.
Suspension Emt Definition at Kathleen Boes blog
Colloid Solution Particle Size Particles this small can neither be seen by the. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. Particles this small can neither be seen by the. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. These particles can be solid, liquid, or gas. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In solutions, particles range in size from 0.01 to one nanometer. In contrast, particles in a solution are smaller than this size, while particles in a. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter. Unlike true solutions where solute particles are dissolved at. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 11.6.1.
From www.researchgate.net
(a) Particle size distribution and (b) Zeta potential of GO colloid at Colloid Solution Particle Size Unlike true solutions where solute particles are dissolved at. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. In contrast, particles in a solution are smaller than this size, while particles in a. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Colloid Solution Particle Size.
From courses.lumenlearning.com
Colloids Chemistry Atoms First Colloid Solution Particle Size These particles can be solid, liquid, or gas. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 11.6.1. Particles this small can neither be seen by the. A colloid is a heterogeneous mixture. Colloid Solution Particle Size.
From www.numerade.com
SOLVED The images depict solid particles of different diameters in a Colloid Solution Particle Size Particles this small can neither be seen by the. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of. Colloid Solution Particle Size.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Colloid Solution Particle Size Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 11.6.1. Particles this small can neither be seen by the. A colloidal solution typically consists of particles ranging in size from. Colloid Solution Particle Size.
From www.pharmaguideline.com
Colloidal dispersions Classification of dispersed systems & their Colloid Solution Particle Size These particles can be solid, liquid, or gas. Unlike true solutions where solute particles are dissolved at. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension.. Colloid Solution Particle Size.
From www.esaral.com
Colloidal Solution Definition Examples Types for Class 12, IITJEE Colloid Solution Particle Size Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Particles this small can neither be seen by the. In contrast, particles in a solution are smaller. Colloid Solution Particle Size.
From dxobhpmpa.blob.core.windows.net
Is A Colloid A Type Of Solution at Kenny Saucedo blog Colloid Solution Particle Size A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 11.6.1. Unlike true solutions where solute particles are dissolved at. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. Particles this small can neither be seen by the. The particles. Colloid Solution Particle Size.
From www.geeksforgeeks.org
What is a Suspension? Colloid Solution Particle Size Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. Particles this small can neither be seen by the. These particles can be solid, liquid, or gas. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 11.6.1. The particles are. Colloid Solution Particle Size.
From pubs.rsc.org
Robust open cellular porous polymer monoliths made from cured colloidal Colloid Solution Particle Size A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In solutions, particles range in size from 0.01 to one nanometer. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between. Colloid Solution Particle Size.
From www.researchgate.net
Particle Size Diameter of Reported Colloids Download Table Colloid Solution Particle Size A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Particles this small can neither be seen by the. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. A colloidal solution typically consists of particles ranging. Colloid Solution Particle Size.
From slideplayer.com
Cells and Their Environment ppt download Colloid Solution Particle Size A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. In contrast, particles in a solution are smaller than this size, while particles in a. A group of mixtures called colloids (or colloidal dispersions). Colloid Solution Particle Size.
From chem.libretexts.org
Colloids Chemistry LibreTexts Colloid Solution Particle Size A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In contrast, particles in a solution are smaller than this size, while particles in a. In solutions, particles range in size from 0.01 to one nanometer. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of. Colloid Solution Particle Size.
From ar.inspiredpencil.com
Colloid Suspension Solution Colloid Solution Particle Size The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter. Particles this small can neither be seen by the. In contrast, particles in a solution are smaller than this size, while particles in a. In solutions, particles range in size from 0.01 to one nanometer. A colloid is a heterogeneous mixture in which. Colloid Solution Particle Size.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Colloid Solution Particle Size Unlike true solutions where solute particles are dissolved at. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 11.6.1. In solutions, particles range in size from 0.01 to one nanometer. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. A colloid is a. Colloid Solution Particle Size.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Colloid Solution Particle Size In solutions, particles range in size from 0.01 to one nanometer. In contrast, particles in a solution are smaller than this size, while particles in a. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Particles. Colloid Solution Particle Size.
From scsscience9.blogspot.com
Is Matter Around Us Pure? Blog 6 Colloid Solution Particle Size The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. These particles can be solid, liquid, or gas. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension. Colloid Solution Particle Size.
From www.vecteezy.com
Different dispersion system, true and colloidal solution and suspension Colloid Solution Particle Size Particles this small can neither be seen by the. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter. A group of mixtures called colloids (or colloidal dispersions) exhibit properties. Colloid Solution Particle Size.
From joislngxm.blob.core.windows.net
Suspension Emt Definition at Kathleen Boes blog Colloid Solution Particle Size In solutions, particles range in size from 0.01 to one nanometer. Particles this small can neither be seen by the. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 11.6.1. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e.. Colloid Solution Particle Size.
From exopyyzzo.blob.core.windows.net
Is Vinegar A Solution Suspension Or Colloid at Valerie Blum blog Colloid Solution Particle Size Unlike true solutions where solute particles are dissolved at. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. In solutions, particles range in size from 0.01 to one nanometer. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 11.6.1.. Colloid Solution Particle Size.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Colloid Solution Particle Size A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Unlike true solutions where solute particles are dissolved at. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. These particles can be solid, liquid, or gas.. Colloid Solution Particle Size.
From www.shutterstock.com
218 vectores de Colloid Vectores, imágenes y arte vectorial de stock Colloid Solution Particle Size Unlike true solutions where solute particles are dissolved at. Particles this small can neither be seen by the. These particles can be solid, liquid, or gas. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloidal. Colloid Solution Particle Size.
From www.purechemistry.org
Determination of the size of Colloidal Particles Purechemistry Colloid Solution Particle Size In solutions, particles range in size from 0.01 to one nanometer. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 11.6.1. In contrast, particles in a solution. Colloid Solution Particle Size.
From 88guru.com
True solution, Suspension, Colloidal Solutions 88Guru Colloid Solution Particle Size A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 11.6.1. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Particles this small can neither be seen by the. A colloidal solution typically consists of particles ranging. Colloid Solution Particle Size.
From slideplayer.com
(solids) Solutions and Other Mixtures ppt download Colloid Solution Particle Size A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. In contrast, particles in a solution are smaller than this size, while particles in a. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. A colloidal. Colloid Solution Particle Size.
From exoegsxur.blob.core.windows.net
Solution Suspension Colloid Quizlet at Mable Finger blog Colloid Solution Particle Size The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter. Particles this small can neither be seen by the. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those. Colloid Solution Particle Size.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Colloid Solution Particle Size In contrast, particles in a solution are smaller than this size, while particles in a. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Unlike true solutions where solute particles are dissolved at. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter. In solutions, particles. Colloid Solution Particle Size.
From ar.inspiredpencil.com
Examples Of Colloid Mixtures Colloid Solution Particle Size Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 11.6.1. These particles can be. Colloid Solution Particle Size.
From pediaa.com
Difference Between Colloid and Solution Definition, Properties Colloid Solution Particle Size In solutions, particles range in size from 0.01 to one nanometer. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. Particles this small can neither be seen by the. In contrast, particles in. Colloid Solution Particle Size.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Colloid Solution Particle Size Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter. In solutions, particles range in size from 0.01 to one nanometer. These particles can be solid, liquid, or gas. Particles this small can. Colloid Solution Particle Size.
From collegedunia.com
Classification of Colloids Properties & Phases Colloid Solution Particle Size A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 11.6.1. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter. These particles can be solid, liquid, or gas. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of. Colloid Solution Particle Size.
From www.vecteezy.com
Coagulation of Colloid Particles vector illustration 21669350 Vector Colloid Solution Particle Size A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 11.6.1. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter. In contrast, particles in a solution are smaller than this size, while particles in a. Colloidal solution is a heterogeneous mixture in which. Colloid Solution Particle Size.
From exyrhbgfo.blob.core.windows.net
What Type Of Colloid Is A Paint at David Tomaszewski blog Colloid Solution Particle Size A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In contrast, particles in a solution are smaller than this size, while particles in a. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Colloidal solution is a heterogeneous mixture. Colloid Solution Particle Size.
From www.slideserve.com
PPT Chapter Twelve Colloidal System and Surface Phenomena PowerPoint Colloid Solution Particle Size Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter. These particles can be solid, liquid, or gas. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1. Colloid Solution Particle Size.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Colloid Solution Particle Size Unlike true solutions where solute particles are dissolved at. These particles can be solid, liquid, or gas. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Colloidal solution is a heterogeneous mixture in which particle size of substance is intermediate of true solution and suspension i.e.. Colloid Solution Particle Size.
From loezijvab.blob.core.windows.net
Colloid Solution Definition at Gary Reese blog Colloid Solution Particle Size Unlike true solutions where solute particles are dissolved at. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Particles this small can neither be seen by the. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1 micrometer (μm) in diameter. A group. Colloid Solution Particle Size.