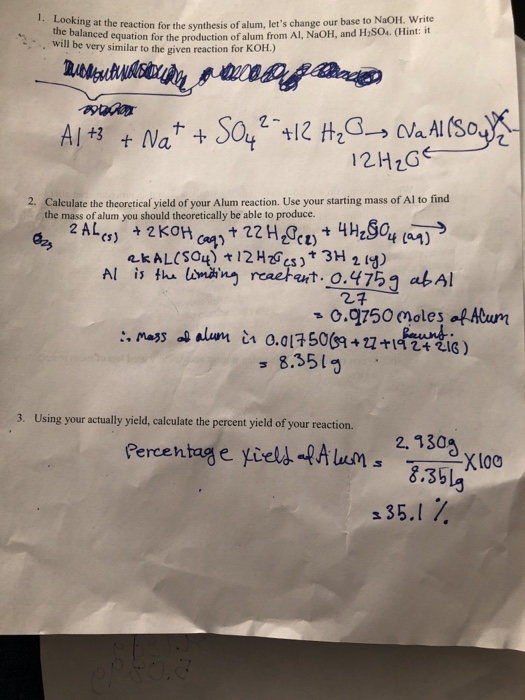Alum And Lime Reaction . When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: Because alum is added in the sample, the reaction is broken down into cations and negative ions. When alum is added, the ph and alkalinity of water tend to decrease. Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. Liming materials react with acidity as follows for dolomite3: 2 = ca2+ + mg2+ + 2h2o + 2co. Lime absorbs moisture from the air, creating hydrated lime [affil links] with a ph of 12.4, which caustic etches the aluminum. When alum is added to water, it reacts with the water and results in positively charged ions. Nearly all coagulant aids are very expensive, so care must be. If the proper chemical control is maintained on lime feed, the calcium hardness may be. Characterisation of the water treatment sludge (wts) generated in drinking water treatment plants (dwtps) is.
from www.chegg.com
Nearly all coagulant aids are very expensive, so care must be. If the proper chemical control is maintained on lime feed, the calcium hardness may be. When alum is added to water, it reacts with the water and results in positively charged ions. Liming materials react with acidity as follows for dolomite3: Because alum is added in the sample, the reaction is broken down into cations and negative ions. Characterisation of the water treatment sludge (wts) generated in drinking water treatment plants (dwtps) is. Lime absorbs moisture from the air, creating hydrated lime [affil links] with a ph of 12.4, which caustic etches the aluminum. When alum is added, the ph and alkalinity of water tend to decrease. Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. 2 = ca2+ + mg2+ + 2h2o + 2co.
Solved reaction for the synthesis of alum, let's change our
Alum And Lime Reaction Nearly all coagulant aids are very expensive, so care must be. 2 = ca2+ + mg2+ + 2h2o + 2co. When alum is added, the ph and alkalinity of water tend to decrease. Nearly all coagulant aids are very expensive, so care must be. Characterisation of the water treatment sludge (wts) generated in drinking water treatment plants (dwtps) is. When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: If the proper chemical control is maintained on lime feed, the calcium hardness may be. Liming materials react with acidity as follows for dolomite3: Because alum is added in the sample, the reaction is broken down into cations and negative ions. When alum is added to water, it reacts with the water and results in positively charged ions. Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. Lime absorbs moisture from the air, creating hydrated lime [affil links] with a ph of 12.4, which caustic etches the aluminum.
From www.chegg.com
Solved 2. 40 mg/L alum dose is applied to a coagulation Alum And Lime Reaction Nearly all coagulant aids are very expensive, so care must be. Liming materials react with acidity as follows for dolomite3: When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: Because alum is added in the sample, the reaction is broken down into cations and negative ions. When alum is added to water,. Alum And Lime Reaction.
From www.semanticscholar.org
[PDF] Evaluation of Alum/Lime Coagulant for the Removal of Turbidity from Al Ahdab Iraqi Alum And Lime Reaction Liming materials react with acidity as follows for dolomite3: When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: When alum is added, the ph and alkalinity of water tend to decrease. Characterisation of the water treatment sludge (wts) generated in drinking water treatment plants (dwtps) is. Lime absorbs moisture from the air,. Alum And Lime Reaction.
From www.scribd.com
Alum Reaction Alkalinity Chemical Elements Alum And Lime Reaction Liming materials react with acidity as follows for dolomite3: When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: Because alum is added in the sample, the reaction is broken down into cations and negative ions. Lime absorbs moisture from the air, creating hydrated lime [affil links] with a ph of 12.4, which. Alum And Lime Reaction.
From ap-chemistry.blogspot.com
AP Chemistry assign and Lab Solutions AP Lab 1 Synthesis of Alum Alum And Lime Reaction When alum is added to water, it reacts with the water and results in positively charged ions. Nearly all coagulant aids are very expensive, so care must be. Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. When alum is added, the ph and alkalinity of water tend to decrease. Characterisation of the water. Alum And Lime Reaction.
From www.youtube.com
Action of Water on Quick Lime Combination Reaction YouTube Alum And Lime Reaction Characterisation of the water treatment sludge (wts) generated in drinking water treatment plants (dwtps) is. If the proper chemical control is maintained on lime feed, the calcium hardness may be. Lime absorbs moisture from the air, creating hydrated lime [affil links] with a ph of 12.4, which caustic etches the aluminum. When alum is added to water, it reacts with. Alum And Lime Reaction.
From www.bartleby.com
Answered 1. In this experiment, aluminum will be… bartleby Alum And Lime Reaction Liming materials react with acidity as follows for dolomite3: Lime absorbs moisture from the air, creating hydrated lime [affil links] with a ph of 12.4, which caustic etches the aluminum. Characterisation of the water treatment sludge (wts) generated in drinking water treatment plants (dwtps) is. When alum is added, the ph and alkalinity of water tend to decrease. Alum, the. Alum And Lime Reaction.
From www.youtube.com
Coagulation using Alum Numericals Water Treatment GATE 2022 Environmental Engineering Alum And Lime Reaction If the proper chemical control is maintained on lime feed, the calcium hardness may be. When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: Because alum is added in the sample, the reaction is broken down into cations and negative ions. When alum is added to water, it reacts with the water. Alum And Lime Reaction.
From www.youtube.com
Chemical Reaction & Equation // Quick lime with water//Exothermic//Sanjay belli//smart learning Alum And Lime Reaction When alum is added to water, it reacts with the water and results in positively charged ions. 2 = ca2+ + mg2+ + 2h2o + 2co. Liming materials react with acidity as follows for dolomite3: Nearly all coagulant aids are very expensive, so care must be. When alum is added, the ph and alkalinity of water tend to decrease. Alum,. Alum And Lime Reaction.
From oneclass.com
OneClass determine the theoretical yield of alum formed in the reaction below. (please show Alum And Lime Reaction 2 = ca2+ + mg2+ + 2h2o + 2co. Liming materials react with acidity as follows for dolomite3: Nearly all coagulant aids are very expensive, so care must be. When alum is added to water, it reacts with the water and results in positively charged ions. If the proper chemical control is maintained on lime feed, the calcium hardness may. Alum And Lime Reaction.
From www.researchgate.net
Measuring the temperature of reaction of lime and aluminum. Download Scientific Diagram Alum And Lime Reaction 2 = ca2+ + mg2+ + 2h2o + 2co. Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. Liming materials react with acidity as follows for dolomite3: Nearly all coagulant aids are very expensive, so care must be. When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions. Alum And Lime Reaction.
From www.chegg.com
Solved Problem No.1 Alum Coagulation An alum dosage of Alum And Lime Reaction 2 = ca2+ + mg2+ + 2h2o + 2co. Because alum is added in the sample, the reaction is broken down into cations and negative ions. Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. Liming materials react with acidity as follows for dolomite3: Nearly all coagulant aids are very expensive, so care must. Alum And Lime Reaction.
From www.researchgate.net
chemical Process of Gypsum and Lime. Download Scientific Diagram Alum And Lime Reaction Nearly all coagulant aids are very expensive, so care must be. 2 = ca2+ + mg2+ + 2h2o + 2co. Characterisation of the water treatment sludge (wts) generated in drinking water treatment plants (dwtps) is. When alum is added, the ph and alkalinity of water tend to decrease. Because alum is added in the sample, the reaction is broken down. Alum And Lime Reaction.
From www.numerade.com
SOLVEDWrite the balanced equation for the reaction of slaked lime (calcium hydroxide) with alum Alum And Lime Reaction Liming materials react with acidity as follows for dolomite3: Because alum is added in the sample, the reaction is broken down into cations and negative ions. Nearly all coagulant aids are very expensive, so care must be. When alum is added to water, it reacts with the water and results in positively charged ions. Alum, the predominant coagulant in conventional. Alum And Lime Reaction.
From www.chegg.com
Solved reaction for the synthesis of alum, let's change our Alum And Lime Reaction If the proper chemical control is maintained on lime feed, the calcium hardness may be. When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: Liming materials react with acidity as follows for dolomite3: 2 = ca2+ + mg2+ + 2h2o + 2co. Nearly all coagulant aids are very expensive, so care must. Alum And Lime Reaction.
From www.numerade.com
SOLVEDSeveral reactions of aluminum are shown in the common reactions section for Group 13 of Alum And Lime Reaction 2 = ca2+ + mg2+ + 2h2o + 2co. Liming materials react with acidity as follows for dolomite3: When alum is added, the ph and alkalinity of water tend to decrease. If the proper chemical control is maintained on lime feed, the calcium hardness may be. Nearly all coagulant aids are very expensive, so care must be. Because alum is. Alum And Lime Reaction.
From www.chegg.com
Solved Exercise 1 A water with low alkalinity of 12 mg/L as Alum And Lime Reaction 2 = ca2+ + mg2+ + 2h2o + 2co. When alum is added, the ph and alkalinity of water tend to decrease. Nearly all coagulant aids are very expensive, so care must be. When alum is added to water, it reacts with the water and results in positively charged ions. If the proper chemical control is maintained on lime feed,. Alum And Lime Reaction.
From sciencing.com
Effects of Lime & Alum on Water Purification Sciencing Alum And Lime Reaction Nearly all coagulant aids are very expensive, so care must be. Lime absorbs moisture from the air, creating hydrated lime [affil links] with a ph of 12.4, which caustic etches the aluminum. When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: Alum, the predominant coagulant in conventional drinking water treatment schemes, has. Alum And Lime Reaction.
From www.numerade.com
SOLVED The synthesis of alum, starting with aluminum, occurs in several steps using four Alum And Lime Reaction Because alum is added in the sample, the reaction is broken down into cations and negative ions. When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: When alum is added to water, it reacts with the water and results in positively charged ions. 2 = ca2+ + mg2+ + 2h2o + 2co.. Alum And Lime Reaction.
From www.hunker.com
Effects of Lime & Alum on Water Purification Hunker Alum And Lime Reaction Because alum is added in the sample, the reaction is broken down into cations and negative ions. When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. If the proper chemical control is maintained on lime feed, the. Alum And Lime Reaction.
From www.youtube.com
Prelab 5 Recycling Aluminum YouTube Alum And Lime Reaction When alum is added, the ph and alkalinity of water tend to decrease. Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. Liming materials react with acidity as follows for dolomite3: When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: 2 = ca2+ + mg2+ +. Alum And Lime Reaction.
From www.researchgate.net
XRD patterns of reaction products of Algoethite and lime in Bayer... Download Scientific Diagram Alum And Lime Reaction Lime absorbs moisture from the air, creating hydrated lime [affil links] with a ph of 12.4, which caustic etches the aluminum. Liming materials react with acidity as follows for dolomite3: When alum is added to water, it reacts with the water and results in positively charged ions. When alum is added, the ph and alkalinity of water tend to decrease.. Alum And Lime Reaction.
From mogckchem.weebly.com
Lime Production from Limestone Current Technology Alum And Lime Reaction Because alum is added in the sample, the reaction is broken down into cations and negative ions. If the proper chemical control is maintained on lime feed, the calcium hardness may be. Nearly all coagulant aids are very expensive, so care must be. Liming materials react with acidity as follows for dolomite3: Alum, the predominant coagulant in conventional drinking water. Alum And Lime Reaction.
From www.youtube.com
Formation of slaked lime using quick lime and water combination reaction class 10 YouTube Alum And Lime Reaction If the proper chemical control is maintained on lime feed, the calcium hardness may be. When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: Nearly all coagulant aids are very expensive, so care must be. When alum is added, the ph and alkalinity of water tend to decrease. Characterisation of the water. Alum And Lime Reaction.
From www.slideserve.com
PPT Jar Testing Coagulation Dosage Water Treatment Plants PowerPoint Presentation ID209724 Alum And Lime Reaction Lime absorbs moisture from the air, creating hydrated lime [affil links] with a ph of 12.4, which caustic etches the aluminum. When alum is added to water, it reacts with the water and results in positively charged ions. Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. When alum is added, the ph and. Alum And Lime Reaction.
From www.researchgate.net
(PDF) Dispersive clay stabilised by alum and lime Alum And Lime Reaction 2 = ca2+ + mg2+ + 2h2o + 2co. Because alum is added in the sample, the reaction is broken down into cations and negative ions. Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. If the proper chemical control is maintained on lime feed, the calcium hardness may be. When hydrated lime, ca. Alum And Lime Reaction.
From dpstar.com.my
Maximizing Water Treatment with Lime & Alum Batching System Alum And Lime Reaction When alum is added to water, it reacts with the water and results in positively charged ions. Liming materials react with acidity as follows for dolomite3: When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: When alum is added, the ph and alkalinity of water tend to decrease. 2 = ca2+ +. Alum And Lime Reaction.
From www.numerade.com
SOLVED EXPERIMENT 9 SYNTHESIS OF ALUM Introduction In this laboratory exercise, alum will be Alum And Lime Reaction When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: 2 = ca2+ + mg2+ + 2h2o + 2co. Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. When alum is added to water, it reacts with the water and results in positively charged ions. Nearly all. Alum And Lime Reaction.
From www.numerade.com
SOLVED Write five balanced equations which correspond to the four intermediate reaction steps Alum And Lime Reaction Characterisation of the water treatment sludge (wts) generated in drinking water treatment plants (dwtps) is. Because alum is added in the sample, the reaction is broken down into cations and negative ions. When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: 2 = ca2+ + mg2+ + 2h2o + 2co. When alum. Alum And Lime Reaction.
From www.youtube.com
How to make a saturated alum solution (part 4) YouTube Alum And Lime Reaction Characterisation of the water treatment sludge (wts) generated in drinking water treatment plants (dwtps) is. When alum is added, the ph and alkalinity of water tend to decrease. Nearly all coagulant aids are very expensive, so care must be. Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. When alum is added to water,. Alum And Lime Reaction.
From www.youtube.com
Activity 1.4 class 10 Science I Reaction of quick lime with water I YouTube Alum And Lime Reaction Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. Because alum is added in the sample, the reaction is broken down into cations and negative ions. Nearly all coagulant aids are very expensive, so care must be. 2 = ca2+ + mg2+ + 2h2o + 2co. When alum is added, the ph and alkalinity. Alum And Lime Reaction.
From www.agric.wa.gov.au
Lime quality Agriculture and Food Alum And Lime Reaction Because alum is added in the sample, the reaction is broken down into cations and negative ions. When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: When alum is added, the ph and alkalinity of water tend to decrease. Lime absorbs moisture from the air, creating hydrated lime [affil links] with a. Alum And Lime Reaction.
From web.deu.edu.tr
Toprak Home Page Alum And Lime Reaction Liming materials react with acidity as follows for dolomite3: Nearly all coagulant aids are very expensive, so care must be. When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. Because alum is added in the sample, the. Alum And Lime Reaction.
From www.researchgate.net
Conversion (mol ) of Lime 3 reaction with HCl at different SO2... Download Scientific Diagram Alum And Lime Reaction Liming materials react with acidity as follows for dolomite3: 2 = ca2+ + mg2+ + 2h2o + 2co. Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. Because alum is added in the sample, the reaction is broken down into cations and negative ions. Lime absorbs moisture from the air, creating hydrated lime [affil. Alum And Lime Reaction.
From www.researchgate.net
Chemical reaction of soil with lime stabilization (After Jaritngam, et.... Download Scientific Alum And Lime Reaction Nearly all coagulant aids are very expensive, so care must be. Alum, the predominant coagulant in conventional drinking water treatment schemes, has various disadvantages including the. Liming materials react with acidity as follows for dolomite3: When alum is added, the ph and alkalinity of water tend to decrease. 2 = ca2+ + mg2+ + 2h2o + 2co. Because alum is. Alum And Lime Reaction.
From www.slideserve.com
PPT Jar Testing Coagulation Dosage Water Treatment Plants PowerPoint Presentation ID209724 Alum And Lime Reaction 2 = ca2+ + mg2+ + 2h2o + 2co. When alum is added to water, it reacts with the water and results in positively charged ions. If the proper chemical control is maintained on lime feed, the calcium hardness may be. When hydrated lime, ca (oh) 2, is added to the water being treated, the following reactions occur: Because alum. Alum And Lime Reaction.