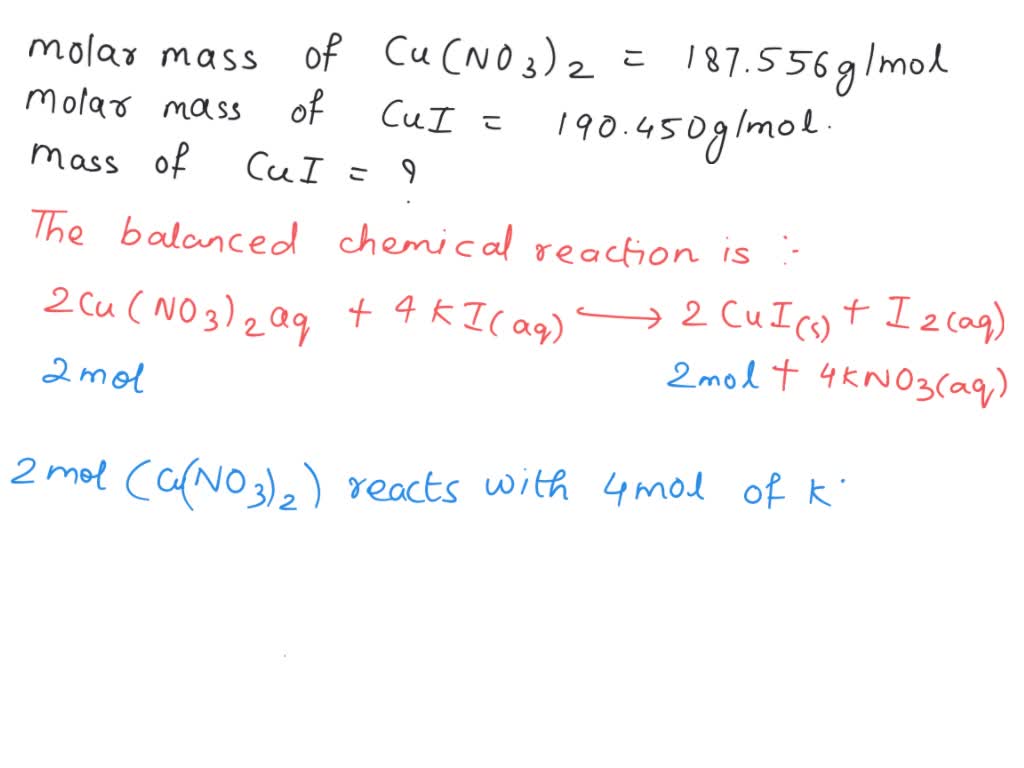How To Make Equation Potassium Iodide . How to write the formula for ki (potassium iodide) in this video we'll write the correct formula. Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide. How do you make potassium iodide? Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot solution. It is used as a. In the first part of the experiment, the rate equation will be determined by investigating the effect of the concentration of. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. First, iodine is reacted with a strong alkali solution, such as. Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together through an ionic. It is an ionic compound that is produced by treating potassium hydroxide (koh) with iodine. It is soluble in water.
from www.animalia-life.club
It is used as a. How to write the formula for ki (potassium iodide) in this video we'll write the correct formula. In the first part of the experiment, the rate equation will be determined by investigating the effect of the concentration of. First, iodine is reacted with a strong alkali solution, such as. It is soluble in water. Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide. How do you make potassium iodide? It is an ionic compound that is produced by treating potassium hydroxide (koh) with iodine. Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together through an ionic. Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot solution.
Potassium Iodide Lewis Dot Structure
How To Make Equation Potassium Iodide It is soluble in water. Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide. Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot solution. In the first part of the experiment, the rate equation will be determined by investigating the effect of the concentration of. How to write the formula for ki (potassium iodide) in this video we'll write the correct formula. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. How do you make potassium iodide? First, iodine is reacted with a strong alkali solution, such as. It is soluble in water. Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together through an ionic. It is used as a. It is an ionic compound that is produced by treating potassium hydroxide (koh) with iodine.
From www.coursehero.com
[Solved] Silver nitrate reacts with potassium iodide in aqueous solution to... Course Hero How To Make Equation Potassium Iodide There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. How to write the formula for ki (potassium iodide) in this video we'll write the correct formula. Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot solution. Write the ionic equation for the reaction of. How To Make Equation Potassium Iodide.
From study.com
Potassium Iodide Formula, Charge & Structure Lesson How To Make Equation Potassium Iodide In the first part of the experiment, the rate equation will be determined by investigating the effect of the concentration of. First, iodine is reacted with a strong alkali solution, such as. Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together through an ionic. There are three main steps. How To Make Equation Potassium Iodide.
From www.animalia-life.club
Potassium Iodide Lewis Dot Structure How To Make Equation Potassium Iodide Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together through an ionic. How do you make potassium iodide? It is used as a. How to write the formula for ki (potassium iodide) in this video we'll write the correct formula. It is an ionic compound that is produced by. How To Make Equation Potassium Iodide.
From www.tessshebaylo.com
Chemical Equation For Hydrogen Peroxide And Potassium Iodide Tessshebaylo How To Make Equation Potassium Iodide It is an ionic compound that is produced by treating potassium hydroxide (koh) with iodine. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide. How to write the formula for ki (potassium iodide) in this. How To Make Equation Potassium Iodide.
From www.youtube.com
Equation for KI + H2O (Potassium iodide + Water) YouTube How To Make Equation Potassium Iodide Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot solution. Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together through an ionic. It is used as a. There are three main steps for writing the net ionic equation for ki + br2 = kbr. How To Make Equation Potassium Iodide.
From www.youtube.com
how to make potassium iodide in laboratory and at home potassium iodide kaise banaye YouTube How To Make Equation Potassium Iodide It is soluble in water. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together through an ionic. In the first part of the experiment, the rate equation will be. How To Make Equation Potassium Iodide.
From www.numerade.com
SOLVED "solid potassium iodide into iodine gas and solid potassium. Write a a How To Make Equation Potassium Iodide It is soluble in water. How do you make potassium iodide? First, iodine is reacted with a strong alkali solution, such as. Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot solution. Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together through an ionic.. How To Make Equation Potassium Iodide.
From kunduz.com
[ANSWERED] Assume that Potassium Iodide and Lead(II) Nitrate are the Kunduz How To Make Equation Potassium Iodide There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. It is soluble in water. It is an ionic compound that is produced by treating potassium hydroxide (koh) with iodine. Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot solution. How do you make potassium. How To Make Equation Potassium Iodide.
From twobirdsfourhands.com
Periodic Table Potassium Iodide Two Birds Home How To Make Equation Potassium Iodide In the first part of the experiment, the rate equation will be determined by investigating the effect of the concentration of. How to write the formula for ki (potassium iodide) in this video we'll write the correct formula. Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide. It is used as a. Potassium iodide is. How To Make Equation Potassium Iodide.
From mavink.com
Potassium Iodide Structure How To Make Equation Potassium Iodide There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot solution. It is soluble in water. First, iodine is reacted with a strong alkali solution, such as. Write the ionic equation for the reaction of aqueous. How To Make Equation Potassium Iodide.
From studylib.net
Preparation of standard potassium iodate(V) solution How To Make Equation Potassium Iodide In the first part of the experiment, the rate equation will be determined by investigating the effect of the concentration of. First, iodine is reacted with a strong alkali solution, such as. Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide. There are three main steps for writing the net ionic equation for ki +. How To Make Equation Potassium Iodide.
From animalia-life.club
Iodine Solid Formula How To Make Equation Potassium Iodide First, iodine is reacted with a strong alkali solution, such as. How do you make potassium iodide? It is an ionic compound that is produced by treating potassium hydroxide (koh) with iodine. It is soluble in water. Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together through an ionic.. How To Make Equation Potassium Iodide.
From www.animalia-life.club
Potassium Iodide Lewis Dot Structure How To Make Equation Potassium Iodide First, iodine is reacted with a strong alkali solution, such as. How do you make potassium iodide? It is soluble in water. Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together through an ionic. How to write the formula for ki (potassium iodide) in this video we'll write the. How To Make Equation Potassium Iodide.
From www.numerade.com
SOLVED 'Reaction of sodium dichromate with potassium iodide in sulfuric acid solution How To Make Equation Potassium Iodide It is soluble in water. Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide. Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot solution. It is an ionic compound that is produced by treating potassium hydroxide (koh) with iodine. In the first part of the experiment, the rate equation will. How To Make Equation Potassium Iodide.
From www.coursehero.com
[Solved] 12.5 g of potassium iodide is placed into enough water to make 100... Course Hero How To Make Equation Potassium Iodide Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot solution. It is used as a. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together. How To Make Equation Potassium Iodide.
From www.tessshebaylo.com
Balanced Chemical Equation For Hydrogen Peroxide And Potassium Iodide Tessshebaylo How To Make Equation Potassium Iodide It is used as a. How to write the formula for ki (potassium iodide) in this video we'll write the correct formula. In the first part of the experiment, the rate equation will be determined by investigating the effect of the concentration of. Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide. It is an. How To Make Equation Potassium Iodide.
From www.coursehero.com
[Solved] 12.5 g of potassium iodide is placed into enough water to make 100... Course Hero How To Make Equation Potassium Iodide First, iodine is reacted with a strong alkali solution, such as. It is an ionic compound that is produced by treating potassium hydroxide (koh) with iodine. Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together through an ionic. There are three main steps for writing the net ionic equation. How To Make Equation Potassium Iodide.
From www.animalia-life.club
Potassium Iodide Lewis Dot Structure How To Make Equation Potassium Iodide It is used as a. It is an ionic compound that is produced by treating potassium hydroxide (koh) with iodine. First, iodine is reacted with a strong alkali solution, such as. How to write the formula for ki (potassium iodide) in this video we'll write the correct formula. There are three main steps for writing the net ionic equation for. How To Make Equation Potassium Iodide.
From proper-cooking.info
Potassium Iodide Solution Formula How To Make Equation Potassium Iodide It is used as a. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. How to write the formula for ki (potassium iodide) in this video we'll write the correct formula. Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide. It is an ionic. How To Make Equation Potassium Iodide.
From www.coursehero.com
[Solved] A chemist mixes aqueous potassium iodide with lead (II) acetate to... Course Hero How To Make Equation Potassium Iodide It is soluble in water. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. In the first part of the experiment, the rate equation will be determined by investigating the effect of the concentration of. Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot. How To Make Equation Potassium Iodide.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview MartLabPro How To Make Equation Potassium Iodide There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. How do you make potassium iodide? First, iodine is reacted with a strong alkali solution, such as. It is soluble in water. In the first part of the experiment, the rate equation will be determined by investigating the effect of. How To Make Equation Potassium Iodide.
From es.slideshare.net
Electrolysis Of Potassium Iodide Solution How To Make Equation Potassium Iodide Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot solution. Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together through an ionic. First, iodine is reacted with a strong alkali solution, such as. It is an ionic compound that is produced by treating potassium. How To Make Equation Potassium Iodide.
From www.animalia-life.club
Potassium Iodide Lewis Dot Structure How To Make Equation Potassium Iodide First, iodine is reacted with a strong alkali solution, such as. It is soluble in water. It is used as a. Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together through an ionic. Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide. There are. How To Make Equation Potassium Iodide.
From www.chegg.com
Solved Solid potassium iodide into solid How To Make Equation Potassium Iodide It is soluble in water. First, iodine is reacted with a strong alkali solution, such as. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. It is an ionic compound that is produced by treating potassium hydroxide (koh) with iodine. Write the ionic equation for the reaction of aqueous. How To Make Equation Potassium Iodide.
From ar.inspiredpencil.com
Potassium Iodide In Water How To Make Equation Potassium Iodide It is an ionic compound that is produced by treating potassium hydroxide (koh) with iodine. In the first part of the experiment, the rate equation will be determined by investigating the effect of the concentration of. It is soluble in water. It is used as a. First, iodine is reacted with a strong alkali solution, such as. There are three. How To Make Equation Potassium Iodide.
From www.slideserve.com
PPT Chapter 4 Chemical Reactions An Introduction PowerPoint Presentation ID602734 How To Make Equation Potassium Iodide How to write the formula for ki (potassium iodide) in this video we'll write the correct formula. How do you make potassium iodide? First, iodine is reacted with a strong alkali solution, such as. It is soluble in water. Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together through. How To Make Equation Potassium Iodide.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview MartLabPro How To Make Equation Potassium Iodide Potassium iodide is a neutral ionic compound that is made of a potassium ion and an iodide ion bonding together through an ionic. It is soluble in water. How to write the formula for ki (potassium iodide) in this video we'll write the correct formula. Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot solution.. How To Make Equation Potassium Iodide.
From www.numerade.com
SOLVED Write the equation for the ofpotassium iodide. If potassium iodide has How To Make Equation Potassium Iodide It is used as a. It is an ionic compound that is produced by treating potassium hydroxide (koh) with iodine. Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot solution. In the first part of the experiment, the rate equation will be determined by investigating the effect of the concentration of. How to write the. How To Make Equation Potassium Iodide.
From ocdrum.com
nickel(ii iodide and potassium carbonate balanced equation) How To Make Equation Potassium Iodide It is soluble in water. It is an ionic compound that is produced by treating potassium hydroxide (koh) with iodine. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. In the first part of the experiment, the rate equation will be determined by investigating the effect of the concentration. How To Make Equation Potassium Iodide.
From www.toppr.com
\"Reaction of potassium iodide solution with lead nitrate solution \" is the example of How To Make Equation Potassium Iodide It is used as a. It is an ionic compound that is produced by treating potassium hydroxide (koh) with iodine. It is soluble in water. First, iodine is reacted with a strong alkali solution, such as. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. Potassium iodide is a. How To Make Equation Potassium Iodide.
From www.youtube.com
Preparation of IodinePotassium iodide solution for starch test YouTube How To Make Equation Potassium Iodide It is an ionic compound that is produced by treating potassium hydroxide (koh) with iodine. In the first part of the experiment, the rate equation will be determined by investigating the effect of the concentration of. Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide. It is soluble in water. How to write the formula. How To Make Equation Potassium Iodide.
From www.youtube.com
How To Make Potassium Iodide YouTube How To Make Equation Potassium Iodide Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide. First, iodine is reacted with a strong alkali solution, such as. Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot solution. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium.. How To Make Equation Potassium Iodide.
From stock.adobe.com
Vecteur Stock Potassium Iodide KI molecule. Simple molecular formula consisting of Potassium How To Make Equation Potassium Iodide How do you make potassium iodide? It is used as a. It is soluble in water. How to write the formula for ki (potassium iodide) in this video we'll write the correct formula. In the first part of the experiment, the rate equation will be determined by investigating the effect of the concentration of. Potassium iodide is a neutral ionic. How To Make Equation Potassium Iodide.
From www.youtube.com
How to Write the Formula for Potassium iodate YouTube How To Make Equation Potassium Iodide Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. Potassium iodide (ki) is prepared by reacting potassium hydroxide to iodine with a hot solution. First, iodine is reacted with a strong alkali solution, such as.. How To Make Equation Potassium Iodide.
From www.youtube.com
how to prepare 10 potassium iodide solution how to make 10 KI solution 10 percent KI How To Make Equation Potassium Iodide It is used as a. It is soluble in water. How to write the formula for ki (potassium iodide) in this video we'll write the correct formula. How do you make potassium iodide? Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide. First, iodine is reacted with a strong alkali solution, such as. Potassium iodide. How To Make Equation Potassium Iodide.