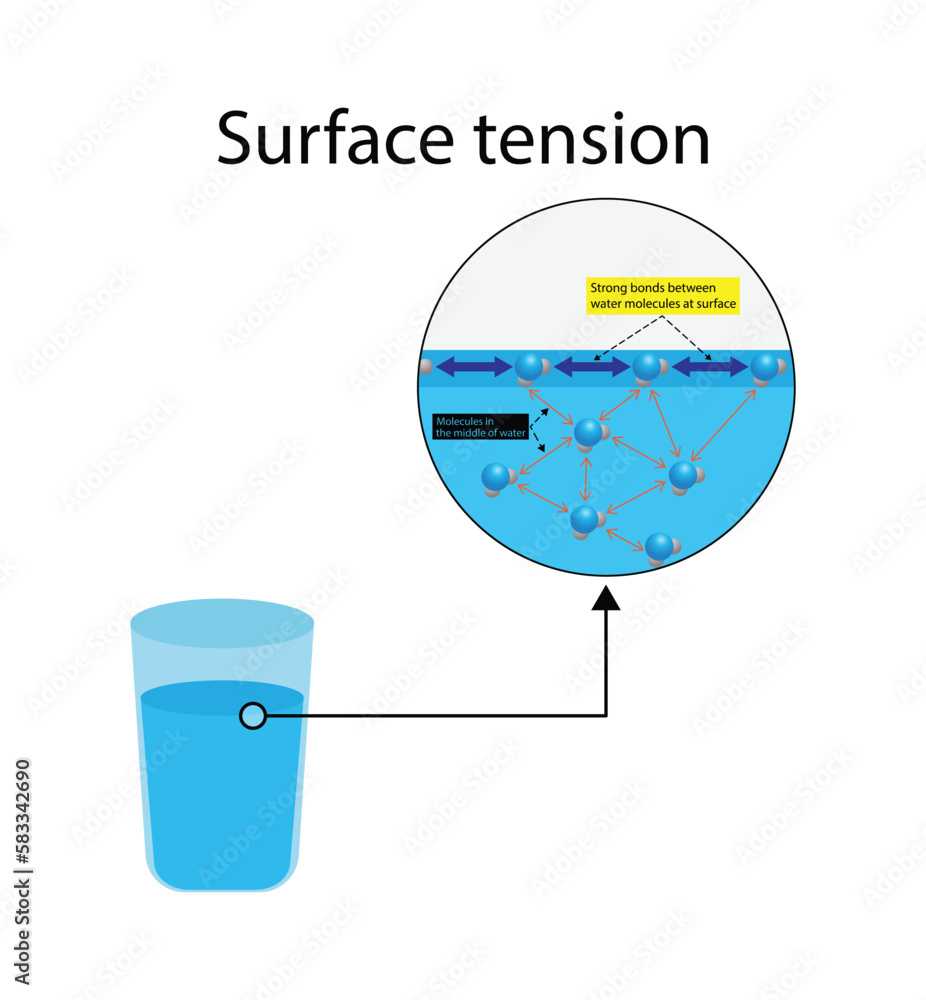
Does Surface Tension Use Adhesion . Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). Surface tension is the reason why liquids form bubbles and droplets. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called surface tension. The surface tension, γ, is the work required to reversibly create a unit area at constant temperature, in units of mj/m 2, mn/m, or dyn/cm. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area.
from stock.adobe.com
This general effect is called surface tension. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The surface tension, γ, is the work required to reversibly create a unit area at constant temperature, in units of mj/m 2, mn/m, or dyn/cm. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). Surface tension is the reason why liquids form bubbles and droplets. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to.
illustration of physics, Surface tension of water, the cohesive forces
Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the reason why liquids form bubbles and droplets. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). This general effect is called surface tension. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The surface tension, γ, is the work required to reversibly create a unit area at constant temperature, in units of mj/m 2, mn/m, or dyn/cm. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area.
From www.youtube.com
Cohesion, Adhesion, & Surface Tension YouTube Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The surface tension, γ, is the work required to reversibly create a unit area at constant temperature, in units of mj/m 2, mn/m, or. Does Surface Tension Use Adhesion.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The surface tension, γ, is the work required to reversibly create a unit area at constant temperature, in units of mj/m 2, mn/m, or. Does Surface Tension Use Adhesion.
From sciencenotes.org
Capillary Action What It Is and How It Works Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to. Surface tension. Does Surface Tension Use Adhesion.
From ar.inspiredpencil.com
The Relationship Between Hydrogen Bonding And Surface Tension, Adhesion Does Surface Tension Use Adhesion Surface tension is the reason why liquids form bubbles and droplets. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). This general effect is called surface tension. Cohesive forces between molecules cause the surface of a liquid to contract to the. Does Surface Tension Use Adhesion.
From pressbooks.online.ucf.edu
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to. This general. Does Surface Tension Use Adhesion.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint Does Surface Tension Use Adhesion This general effect is called surface tension. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to. Surface tension is caused by a strong attraction between the molecules (cohesion) that. Does Surface Tension Use Adhesion.
From www.youtube.com
Surface Tension and Adhesion Fluids Physics Khan Academy YouTube Does Surface Tension Use Adhesion Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the. Does Surface Tension Use Adhesion.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). Cohesive forces between molecules cause the surface of a liquid to contract to the. Does Surface Tension Use Adhesion.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Does Surface Tension Use Adhesion The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). This general effect is called surface tension. Cohesive forces between. Does Surface Tension Use Adhesion.
From www.youtube.com
Adhesion, Cohesion and Surface Tension Part 10 YouTube Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). Cohesive forces between molecules cause the surface of a liquid to contract to the. Does Surface Tension Use Adhesion.
From pressbooks.uiowa.edu
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed. Does Surface Tension Use Adhesion.
From www.slideshare.net
Properties of Water PowerPoint, Adhesion, Cohesion, Surface Tension Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called surface tension. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link. Does Surface Tension Use Adhesion.
From www.youtube.com
Surface Tension and Adhesion Fluids Physics Khan Academy HD YouTube Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to. This general. Does Surface Tension Use Adhesion.
From www.studypool.com
SOLUTION Cohesion adhesion surface tension Studypool Does Surface Tension Use Adhesion Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the. Does Surface Tension Use Adhesion.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download Does Surface Tension Use Adhesion Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the reason why liquids form bubbles and droplets. This general effect. Does Surface Tension Use Adhesion.
From slideplayer.com
Chemical Properties of Water ppt download Does Surface Tension Use Adhesion This general effect is called surface tension. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to. Cohesive forces between molecules cause the surface of a liquid to contract to. Does Surface Tension Use Adhesion.
From www.slideserve.com
PPT Cohesion, Adhesion, and Surface Tension PowerPoint Presentation Does Surface Tension Use Adhesion Surface tension is the reason why liquids form bubbles and droplets. This general effect is called surface tension. The surface tension, γ, is the work required to reversibly create a unit area at constant temperature, in units of mj/m 2, mn/m, or dyn/cm. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the. Does Surface Tension Use Adhesion.
From www.slideserve.com
PPT Understanding Water PowerPoint Presentation, free download ID Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The surface tension, γ, is the work required to reversibly create a unit area at constant temperature, in units of mj/m 2, mn/m, or. Does Surface Tension Use Adhesion.
From www.slideserve.com
PPT EXAM I Powerpoint II A Little Chemistry PowerPoint Presentation Does Surface Tension Use Adhesion Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The surface tension, γ, is the work required to reversibly create a unit area. Does Surface Tension Use Adhesion.
From www.youtube.com
Chemistry Explained Viscosity, Surface Tension, Adhesion, Cohesion Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called surface tension. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link. Does Surface Tension Use Adhesion.
From www.laserax.com
How to Do Surface Preparation for Adhesive Bonding Laserax Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to. This general effect is called surface tension. Cohesive forces between molecules cause the surface of a liquid to contract to. Does Surface Tension Use Adhesion.
From www.expii.com
Cohesion and Adhesion — Definition & Overview Expii Does Surface Tension Use Adhesion The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). Cohesive forces between molecules cause the surface of a liquid. Does Surface Tension Use Adhesion.
From www.slideserve.com
PPT Notes Water and its Special Properties PowerPoint Presentation Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the reason why liquids form bubbles and droplets. The surface tension, γ, is the work required to reversibly create a unit. Does Surface Tension Use Adhesion.
From www.atlanticgasket.com
3M Adhesion Basics Atlantic Gasket Corporation Does Surface Tension Use Adhesion Surface tension is the reason why liquids form bubbles and droplets. This general effect is called surface tension. The surface tension, γ, is the work required to reversibly create a unit area at constant temperature, in units of mj/m 2, mn/m, or dyn/cm. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface. Does Surface Tension Use Adhesion.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID5692577 Does Surface Tension Use Adhesion Surface tension is the reason why liquids form bubbles and droplets. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The surface tension, γ, is the work required to reversibly create a unit area at constant temperature, in units of mj/m 2, mn/m, or dyn/cm. Cohesive forces between molecules cause the. Does Surface Tension Use Adhesion.
From courses.lumenlearning.com
Cohesion and Adhesion in Liquids Surface Tension and Capillary Action Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain. Does Surface Tension Use Adhesion.
From www.celadon.com.tw
Intruction of Adhesive Tape The Science of Adhesive Tapes Precision Does Surface Tension Use Adhesion This general effect is called surface tension. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to. Does Surface Tension Use Adhesion.
From dokumen.tips
(PPT) Lecture 9 Surface Energy, Surface tension and Adhesion energy Does Surface Tension Use Adhesion Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). This general effect is called surface tension. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to. Cohesive forces between. Does Surface Tension Use Adhesion.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint Does Surface Tension Use Adhesion Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). The surface tension, γ, is the work required to reversibly create a unit area at constant temperature, in units of mj/m 2, mn/m, or dyn/cm. Cohesive forces between molecules cause the surface. Does Surface Tension Use Adhesion.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called surface tension. Surface tension is the reason why liquids form bubbles and droplets. Cohesive forces between molecules cause the. Does Surface Tension Use Adhesion.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint Does Surface Tension Use Adhesion The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). Cohesive forces between molecules cause the surface of a liquid. Does Surface Tension Use Adhesion.
From byjus.com
Explain the surface tension phenomenon with examples. Does Surface Tension Use Adhesion This general effect is called surface tension. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). Surface tension is the reason why liquids form bubbles and droplets. Cohesive forces between molecules cause the surface of a liquid to contract to the. Does Surface Tension Use Adhesion.
From technology.techwallp.xyz
Water and its properties online presentation Does Surface Tension Use Adhesion The surface tension, γ, is the work required to reversibly create a unit area at constant temperature, in units of mj/m 2, mn/m, or dyn/cm. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link. Does Surface Tension Use Adhesion.
From slideplayer.com
Basics of Chemistry Biology 9/22/ ppt download Does Surface Tension Use Adhesion Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the reason why liquids form bubbles and droplets. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). This general effect. Does Surface Tension Use Adhesion.
From ar.inspiredpencil.com
The Relationship Between Hydrogen Bonding And Surface Tension, Adhesion Does Surface Tension Use Adhesion The surface tension, γ, is the work required to reversibly create a unit area at constant temperature, in units of mj/m 2, mn/m, or dyn/cm. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to. Cohesive forces between molecules cause the surface of a liquid to contract to. Does Surface Tension Use Adhesion.