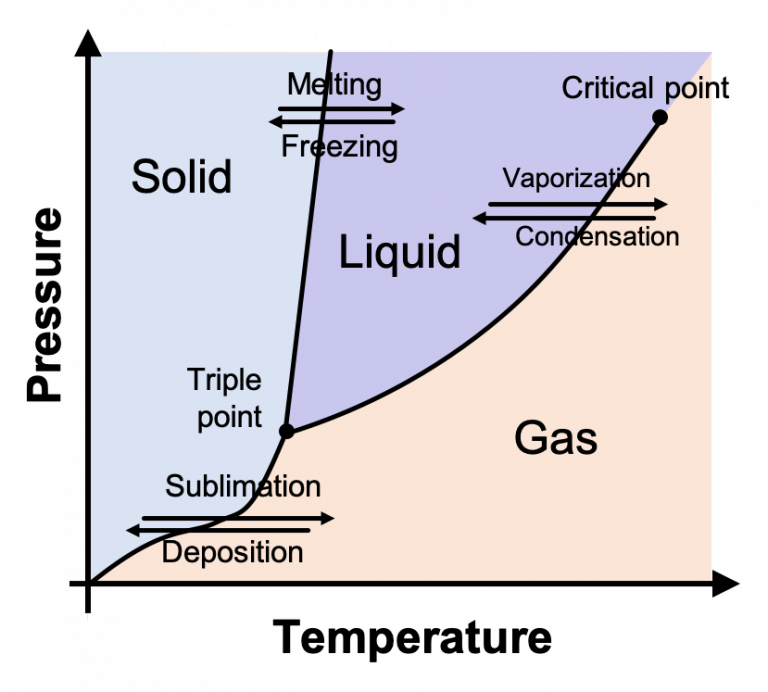
How Does Air Pressure Affect Phase Changes . Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. The existence of the three phases with respect to pressure and temperature can be described. In the picture above, we have a container fitted with a piston. Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. Heats of fusion and vaporization 1. Describe the relationship between heat (energy), bonding forces, and phase changes. Phase changes among the various phases of matter depend on temperature and pressure. Changes of state are examples of phase. The existence of the three phases with respect to. Phase changes among the various phases of matter depend on temperature and pressure. Pressure can also be used to change the phase of the substance.
from wisc.pb.unizin.org
In the picture above, we have a container fitted with a piston. Describe the relationship between heat (energy), bonding forces, and phase changes. The existence of the three phases with respect to. Phase changes among the various phases of matter depend on temperature and pressure. The existence of the three phases with respect to pressure and temperature can be described. Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Changes of state are examples of phase. Heats of fusion and vaporization 1. Phase changes among the various phases of matter depend on temperature and pressure.
Features of Phase Diagrams (M11Q1) UWMadison Chemistry 103/104
How Does Air Pressure Affect Phase Changes Pressure can also be used to change the phase of the substance. Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. Changes of state are examples of phase. Describe the relationship between heat (energy), bonding forces, and phase changes. Pressure can also be used to change the phase of the substance. In the picture above, we have a container fitted with a piston. Phase changes among the various phases of matter depend on temperature and pressure. Heats of fusion and vaporization 1. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. The existence of the three phases with respect to pressure and temperature can be described. Phase changes among the various phases of matter depend on temperature and pressure. The existence of the three phases with respect to.
From courses.lumenlearning.com
Phase Diagrams Chemistry How Does Air Pressure Affect Phase Changes Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. Phase changes among the various phases of matter depend on temperature and pressure. The existence of the three phases with respect to pressure and temperature can be described. The existence of the three phases with respect to. Fusion, vaporization, and. How Does Air Pressure Affect Phase Changes.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/ How Does Air Pressure Affect Phase Changes The existence of the three phases with respect to. Pressure can also be used to change the phase of the substance. The existence of the three phases with respect to pressure and temperature can be described. Changes of state are examples of phase. Heats of fusion and vaporization 1. Describe the relationship between heat (energy), bonding forces, and phase changes.. How Does Air Pressure Affect Phase Changes.
From guidemanualcoset.z21.web.core.windows.net
How To Read A Phase Change Diagram How Does Air Pressure Affect Phase Changes The existence of the three phases with respect to pressure and temperature can be described. Describe the relationship between heat (energy), bonding forces, and phase changes. Heats of fusion and vaporization 1. In the picture above, we have a container fitted with a piston. The existence of the three phases with respect to. Phase changes among the various phases of. How Does Air Pressure Affect Phase Changes.
From www.slideserve.com
PPT Air Pressure and Wind PowerPoint Presentation, free download ID How Does Air Pressure Affect Phase Changes Describe the relationship between heat (energy), bonding forces, and phase changes. Phase changes among the various phases of matter depend on temperature and pressure. Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. Pressure can also be used to change the phase of the substance. Changes of state are. How Does Air Pressure Affect Phase Changes.
From courses.lumenlearning.com
Phase Changes Physics How Does Air Pressure Affect Phase Changes Describe the relationship between heat (energy), bonding forces, and phase changes. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. The existence of the three phases with respect to. Phase changes among the various phases of matter depend on temperature and pressure. Pressure can also be used to change the phase of the substance.. How Does Air Pressure Affect Phase Changes.
From www.britannica.com
phase Definition & Facts Britannica How Does Air Pressure Affect Phase Changes Heats of fusion and vaporization 1. Describe the relationship between heat (energy), bonding forces, and phase changes. The existence of the three phases with respect to. Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. Phase changes among the various phases of matter depend on temperature and pressure. In. How Does Air Pressure Affect Phase Changes.
From www.sliderbase.com
Phase Diagrams Presentation Chemistry How Does Air Pressure Affect Phase Changes Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Phase changes among the various phases of matter depend on temperature and pressure. Pressure can also be used to change the phase of the substance. The existence of the three phases with respect to pressure and temperature can be described. In the picture above, we. How Does Air Pressure Affect Phase Changes.
From www.pinterest.com
Atmosphere characteristics, air pressure Molecule diagram, Earth and How Does Air Pressure Affect Phase Changes Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. The existence of the three phases with respect to. Changes of state are examples of phase. Heats of fusion and vaporization 1. Describe the relationship between heat (energy), bonding forces, and phase changes. Pressure can also be used to change. How Does Air Pressure Affect Phase Changes.
From unistudium.unipg.it
Phase Diagrams How Does Air Pressure Affect Phase Changes The existence of the three phases with respect to pressure and temperature can be described. Describe the relationship between heat (energy), bonding forces, and phase changes. Pressure can also be used to change the phase of the substance. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Phase changes among the various phases of. How Does Air Pressure Affect Phase Changes.
From courses.lumenlearning.com
Solid to Gas Phase Transition Introduction to Chemistry How Does Air Pressure Affect Phase Changes Phase changes among the various phases of matter depend on temperature and pressure. Pressure can also be used to change the phase of the substance. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Changes of state are examples of phase. In the picture above, we have a container fitted with a piston. Phase. How Does Air Pressure Affect Phase Changes.
From www.reddit.com
What is the effect of air pressure on melting point of water? r How Does Air Pressure Affect Phase Changes Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. The existence of the three phases with respect to pressure and temperature can be described. Heats of fusion and vaporization 1. Phase changes among the various phases of matter depend on temperature and pressure. The existence of the three phases. How Does Air Pressure Affect Phase Changes.
From materialdbhutchins.z21.web.core.windows.net
Heat During Phase Change Formula How Does Air Pressure Affect Phase Changes In the picture above, we have a container fitted with a piston. Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. Heats of fusion and vaporization 1. Phase changes among the various phases of matter depend on temperature and pressure. Pressure can also be used to change the phase. How Does Air Pressure Affect Phase Changes.
From www.researchgate.net
1 The atmospheric density and pressure distribution. Download How Does Air Pressure Affect Phase Changes Describe the relationship between heat (energy), bonding forces, and phase changes. Heats of fusion and vaporization 1. Phase changes among the various phases of matter depend on temperature and pressure. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Pressure can also be used to change the phase of the substance. The existence of. How Does Air Pressure Affect Phase Changes.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii How Does Air Pressure Affect Phase Changes In the picture above, we have a container fitted with a piston. Pressure can also be used to change the phase of the substance. Describe the relationship between heat (energy), bonding forces, and phase changes. The existence of the three phases with respect to. Changes of state are examples of phase. Phase changes can have a tremendous stabilizing effect even. How Does Air Pressure Affect Phase Changes.
From joidcbkoc.blob.core.windows.net
Air Pressure Change Last 24 Hours at Kevin Kirby blog How Does Air Pressure Affect Phase Changes Pressure can also be used to change the phase of the substance. In the picture above, we have a container fitted with a piston. The existence of the three phases with respect to pressure and temperature can be described. Phase changes among the various phases of matter depend on temperature and pressure. Describe the relationship between heat (energy), bonding forces,. How Does Air Pressure Affect Phase Changes.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical How Does Air Pressure Affect Phase Changes In the picture above, we have a container fitted with a piston. Phase changes among the various phases of matter depend on temperature and pressure. Heats of fusion and vaporization 1. Changes of state are examples of phase. Pressure can also be used to change the phase of the substance. The existence of the three phases with respect to. Phase. How Does Air Pressure Affect Phase Changes.
From studyrocket.co.uk
Turning Forces and Pressure GCSE Physics AQA Revision Study Rocket How Does Air Pressure Affect Phase Changes Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. The existence of the three phases with respect to. Phase changes among the various phases of matter depend on temperature and pressure. Describe the relationship between heat (energy), bonding forces, and phase changes. Changes of state are examples of phase. Phase changes can have a. How Does Air Pressure Affect Phase Changes.
From klaocdyzj.blob.core.windows.net
How Do Air Pressure And Temperature Change As Altitude Increases at Joy How Does Air Pressure Affect Phase Changes In the picture above, we have a container fitted with a piston. Phase changes among the various phases of matter depend on temperature and pressure. Pressure can also be used to change the phase of the substance. Changes of state are examples of phase. The existence of the three phases with respect to. Heats of fusion and vaporization 1. Phase. How Does Air Pressure Affect Phase Changes.
From studylib.net
How Does Temperature Affect Air Pressure and Wind? How Does Air Pressure Affect Phase Changes Phase changes among the various phases of matter depend on temperature and pressure. Phase changes among the various phases of matter depend on temperature and pressure. In the picture above, we have a container fitted with a piston. Pressure can also be used to change the phase of the substance. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation,. How Does Air Pressure Affect Phase Changes.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical How Does Air Pressure Affect Phase Changes Phase changes among the various phases of matter depend on temperature and pressure. Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. The existence of the three phases with respect to. Phase changes among the various phases of matter depend on temperature and pressure. Fusion, vaporization, and sublimation are. How Does Air Pressure Affect Phase Changes.
From socratic.org
What is the relation between critical temperature and boiling point or How Does Air Pressure Affect Phase Changes Phase changes among the various phases of matter depend on temperature and pressure. Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. Heats of fusion and vaporization 1. Describe the relationship between heat (energy), bonding forces, and phase changes. Pressure can also be used to change the phase of. How Does Air Pressure Affect Phase Changes.
From lavelle.chem.ucla.edu
melting CHEMISTRY COMMUNITY How Does Air Pressure Affect Phase Changes Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. Phase changes among the various phases of matter depend on temperature and pressure. In the picture above, we have a container fitted with a piston. The existence of the three phases with respect to pressure and temperature can be described.. How Does Air Pressure Affect Phase Changes.
From www.unm.edu
BIOL 238 Class Notes Respiratory System How Does Air Pressure Affect Phase Changes The existence of the three phases with respect to. In the picture above, we have a container fitted with a piston. Heats of fusion and vaporization 1. Pressure can also be used to change the phase of the substance. Changes of state are examples of phase. Phase changes among the various phases of matter depend on temperature and pressure. Describe. How Does Air Pressure Affect Phase Changes.
From www.slideshare.net
Phase changes How Does Air Pressure Affect Phase Changes Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. Phase changes among the various phases of matter depend on temperature and pressure. The existence of the three phases with respect to pressure and temperature can be described. Changes of state are examples of phase. Fusion, vaporization, and sublimation are. How Does Air Pressure Affect Phase Changes.
From scied.ucar.edu
Change in the Atmosphere with Altitude Center for Science Education How Does Air Pressure Affect Phase Changes Heats of fusion and vaporization 1. In the picture above, we have a container fitted with a piston. Describe the relationship between heat (energy), bonding forces, and phase changes. The existence of the three phases with respect to. Phase changes among the various phases of matter depend on temperature and pressure. Changes of state are examples of phase. Pressure can. How Does Air Pressure Affect Phase Changes.
From www.slideserve.com
PPT Water and Air Pressure PowerPoint Presentation, free download How Does Air Pressure Affect Phase Changes In the picture above, we have a container fitted with a piston. Phase changes among the various phases of matter depend on temperature and pressure. Describe the relationship between heat (energy), bonding forces, and phase changes. Heats of fusion and vaporization 1. Pressure can also be used to change the phase of the substance. Fusion, vaporization, and sublimation are endothermic. How Does Air Pressure Affect Phase Changes.
From www.chemistrylearner.com
Phase Diagram Definition, Explanation, and Diagram How Does Air Pressure Affect Phase Changes The existence of the three phases with respect to. Phase changes among the various phases of matter depend on temperature and pressure. Phase changes among the various phases of matter depend on temperature and pressure. Changes of state are examples of phase. Heats of fusion and vaporization 1. The existence of the three phases with respect to pressure and temperature. How Does Air Pressure Affect Phase Changes.
From wisc.pb.unizin.org
Features of Phase Diagrams (M11Q1) UWMadison Chemistry 103/104 How Does Air Pressure Affect Phase Changes Describe the relationship between heat (energy), bonding forces, and phase changes. Heats of fusion and vaporization 1. Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. In the picture above, we have a container. How Does Air Pressure Affect Phase Changes.
From chem.libretexts.org
5.6 Phase Diagrams Chemistry LibreTexts How Does Air Pressure Affect Phase Changes The existence of the three phases with respect to pressure and temperature can be described. Describe the relationship between heat (energy), bonding forces, and phase changes. Heats of fusion and vaporization 1. In the picture above, we have a container fitted with a piston. Phase changes among the various phases of matter depend on temperature and pressure. Phase changes among. How Does Air Pressure Affect Phase Changes.
From en.ppt-online.org
Air Pressure and Hot Air Balloons online presentation How Does Air Pressure Affect Phase Changes Phase changes among the various phases of matter depend on temperature and pressure. Changes of state are examples of phase. Heats of fusion and vaporization 1. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. The existence of the three phases with respect to pressure and temperature can be described. Pressure can also be. How Does Air Pressure Affect Phase Changes.
From general.chemistrysteps.com
Entropy and State Change Chemistry Steps How Does Air Pressure Affect Phase Changes Changes of state are examples of phase. Heats of fusion and vaporization 1. Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. The existence of the three phases with respect to pressure and temperature can be described. Describe the relationship between heat (energy), bonding forces, and phase changes. Phase. How Does Air Pressure Affect Phase Changes.
From sciencepickle.com
Latent Heat Science Pickle How Does Air Pressure Affect Phase Changes The existence of the three phases with respect to pressure and temperature can be described. Describe the relationship between heat (energy), bonding forces, and phase changes. Changes of state are examples of phase. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Phase changes among the various phases of matter depend on temperature and. How Does Air Pressure Affect Phase Changes.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo How Does Air Pressure Affect Phase Changes Describe the relationship between heat (energy), bonding forces, and phase changes. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. The existence of the three phases with respect to. Phase changes among the various phases of matter depend on temperature and pressure. Heats of fusion and vaporization 1. Changes of state are examples of. How Does Air Pressure Affect Phase Changes.
From quizlet.com
Unit 4 Phase change and graph Diagram Quizlet How Does Air Pressure Affect Phase Changes In the picture above, we have a container fitted with a piston. Heats of fusion and vaporization 1. Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. Phase changes among the various phases of matter depend on temperature and pressure. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing,. How Does Air Pressure Affect Phase Changes.
From learnject.blogspot.com
Atmospheric Pressure and Its Effects on Human Physiology How Does Air Pressure Affect Phase Changes Heats of fusion and vaporization 1. Pressure can also be used to change the phase of the substance. Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points,. The existence of the three phases with respect to. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are. How Does Air Pressure Affect Phase Changes.