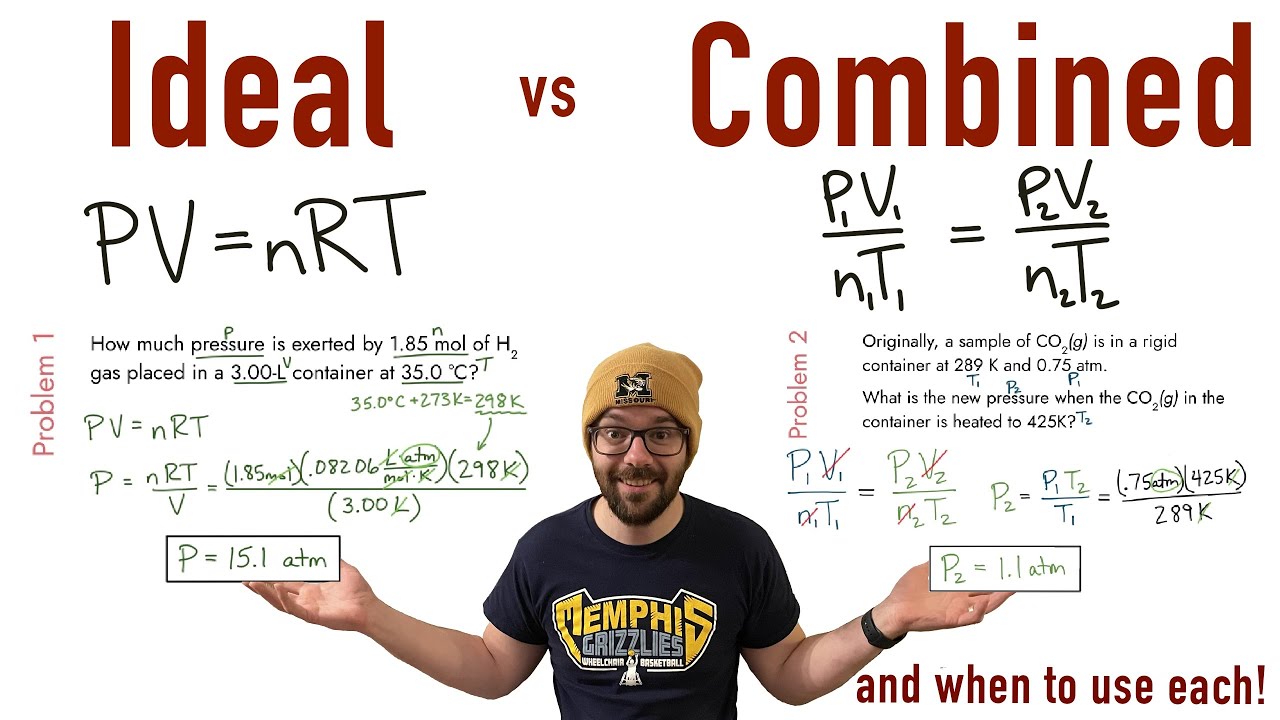
Ap Chemistry Lab Gas Laws . Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. Using the data provided through the procedures, the numerical value of the pressure, volume, gas. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). Ap chem guide's crash course on the ideal gas law. The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. Gas laws summary ap chemistry summary #1 1. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed.
from www.youtube.com
Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Gas laws summary ap chemistry summary #1 1. Using the data provided through the procedures, the numerical value of the pressure, volume, gas. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. Ap chem guide's crash course on the ideal gas law.
Ideal and Combined Gas Laws + When to use them! (AP Chemistry) YouTube
Ap Chemistry Lab Gas Laws Using the data provided through the procedures, the numerical value of the pressure, volume, gas. Ap chem guide's crash course on the ideal gas law. Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. Using the data provided through the procedures, the numerical value of the pressure, volume, gas. Gas laws summary ap chemistry summary #1 1. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as.
From www.studocu.com
Gas Laws Lab Sheet lab Gas Laws Lab Sheet Sarah Carrasquillo, John Ap Chemistry Lab Gas Laws To find the moles, it was essential to utilize the ideal gas law (pv = nrt). Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Using the data provided through the procedures, the numerical value of the pressure, volume, gas. Use the rearranged ideal gas law given in the background to. Ap Chemistry Lab Gas Laws.
From www.youtube.com
Ideal and Combined Gas Laws + When to use them! (AP Chemistry) YouTube Ap Chemistry Lab Gas Laws Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). Gas laws summary ap chemistry summary #1 1. Using the data provided through the procedures, the numerical value of the pressure, volume, gas. Use the rearranged. Ap Chemistry Lab Gas Laws.
From blog.prepscholar.com
AP Chem Formula Sheet What's on It and How to Use It · PrepScholar Ap Chemistry Lab Gas Laws The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Ap chem guide's crash course on the ideal gas law. Using the data provided through the procedures, the numerical. Ap Chemistry Lab Gas Laws.
From www.albert.io
Ideal Gas Law AP® Chemistry Crash Course Review Albert.io Ap Chemistry Lab Gas Laws Gas laws summary ap chemistry summary #1 1. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). Ap chem guide's crash course on the ideal gas law. Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. Using the data. Ap Chemistry Lab Gas Laws.
From www.studyxapp.com
chemistry ideal gas law constant introduction laboratory simulation a Ap Chemistry Lab Gas Laws Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. Ap chem guide's crash course on the ideal gas law. Gas laws summary ap chemistry summary #1 1. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. To. Ap Chemistry Lab Gas Laws.
From www.youtube.com
AP Chemistry Ideal Gas Law YouTube Ap Chemistry Lab Gas Laws Using the data provided through the procedures, the numerical value of the pressure, volume, gas. The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Ap chem guide's crash. Ap Chemistry Lab Gas Laws.
From www.youtube.com
Leggett AP Chem TOT 10 IDEAL GAS LAW.mp4 YouTube Ap Chemistry Lab Gas Laws Ap chem guide's crash course on the ideal gas law. Gas laws summary ap chemistry summary #1 1. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. Using the data. Ap Chemistry Lab Gas Laws.
From studylib.net
Study Guide for AP Chemistry Chapter 5, Gas Laws Ap Chemistry Lab Gas Laws Using the data provided through the procedures, the numerical value of the pressure, volume, gas. Ap chem guide's crash course on the ideal gas law. The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. To find the moles, it was essential to utilize the ideal gas. Ap Chemistry Lab Gas Laws.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Ap Chemistry Lab Gas Laws The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). Using the data provided through the procedures, the numerical value of the pressure, volume, gas. Use the rearranged ideal gas law. Ap Chemistry Lab Gas Laws.
From www.chegg.com
Honors Chemistry LABGas Laws Simulation Name Per Ap Chemistry Lab Gas Laws The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. Using the data provided through the procedures, the numerical value of the pressure, volume, gas. Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each. Ap Chemistry Lab Gas Laws.
From www.studocu.com
Lab+6 gas laws lab Principles of Chemistry Lab 5 Properties of Ap Chemistry Lab Gas Laws Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Using the data provided through the procedures, the numerical value of the pressure, volume, gas. Gas laws summary ap. Ap Chemistry Lab Gas Laws.
From zakruti.com
Worked example Using the ideal gas law to calculate number of moles AP Ap Chemistry Lab Gas Laws Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Gas laws summary ap chemistry summary #1 1. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles. Ap Chemistry Lab Gas Laws.
From oertx.highered.texas.gov
General Chemistry for Science Majors, Unit 3, Gas Laws OERTX Ap Chemistry Lab Gas Laws Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. Ap chem guide's crash course on the ideal gas law. Gas laws summary ap chemistry summary #1 1. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. To. Ap Chemistry Lab Gas Laws.
From www.studypool.com
SOLUTION College general chemistry gas laws tutorial and answer key Ap Chemistry Lab Gas Laws Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of. Ap Chemistry Lab Gas Laws.
From larajackson.z13.web.core.windows.net
Ap Chemistry Unit 3 Ap Chemistry Lab Gas Laws Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. Ap chem guide's crash course on the ideal gas law. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). The equations that express the relationships among t (temperature), p (pressure),. Ap Chemistry Lab Gas Laws.
From glancychem.com
Chemistry Resources 3 GlancyChem Ap Chemistry Lab Gas Laws Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. Ap chem guide's crash course on the ideal gas law. To find the moles, it was essential to utilize. Ap Chemistry Lab Gas Laws.
From www.youtube.com
Gas Law Formulas and Equations College Chemistry Study Guide YouTube Ap Chemistry Lab Gas Laws Gas laws summary ap chemistry summary #1 1. The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. Ap chem guide's crash course on. Ap Chemistry Lab Gas Laws.
From mfawriting332.web.fc2.com
Ap Chemistry Ideal Gas Law Problems 2 Ap Chemistry Lab Gas Laws Gas laws summary ap chemistry summary #1 1. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). Using the data provided through the procedures, the numerical value of the pressure, volume, gas. The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are. Ap Chemistry Lab Gas Laws.
From www.studypool.com
SOLUTION Ideal gas law worksheet 2 answer Studypool Ap Chemistry Lab Gas Laws The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. Ap chem guide's crash course on the ideal gas law. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. To find the moles, it was essential to utilize. Ap Chemistry Lab Gas Laws.
From www.studocu.com
Ap chem gas laws problems AP CHEM Gas Laws Worksheet Use the ideal Ap Chemistry Lab Gas Laws The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for. Ap Chemistry Lab Gas Laws.
From www.studocu.com
Gas Laws Lab Sheet Lab Gas Laws Lab Sheet Pre lab 1. After reading Ap Chemistry Lab Gas Laws To find the moles, it was essential to utilize the ideal gas law (pv = nrt). Using the data provided through the procedures, the numerical value of the pressure, volume, gas. The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. Gas laws summary ap chemistry summary. Ap Chemistry Lab Gas Laws.
From www.homeworkminutes.com
What are the Different Gas Laws in Chemistry Ap Chemistry Lab Gas Laws To find the moles, it was essential to utilize the ideal gas law (pv = nrt). The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. Gas laws summary ap chemistry summary #1 1. Using the data provided through the procedures, the numerical value of the pressure,. Ap Chemistry Lab Gas Laws.
From studylib.net
AP CHEM NOTES GAS LAWS Ap Chemistry Lab Gas Laws Gas laws summary ap chemistry summary #1 1. Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Using the data provided through the procedures, the numerical value of. Ap Chemistry Lab Gas Laws.
From www.youtube.com
"Pressure, Gas Laws, & The Ideal Gas Equation" AP Chemistry with Ap Chemistry Lab Gas Laws Gas laws summary ap chemistry summary #1 1. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). Using the data provided through the procedures, the numerical value of the pressure, volume, gas. Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for. Ap Chemistry Lab Gas Laws.
From www.pinterest.com
Pin by Jessica Joyce on Ideal Gas Law Ideal gas law, Gas laws Ap Chemistry Lab Gas Laws Gas laws summary ap chemistry summary #1 1. Using the data provided through the procedures, the numerical value of the pressure, volume, gas. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of. Ap Chemistry Lab Gas Laws.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 Ap Chemistry Lab Gas Laws Using the data provided through the procedures, the numerical value of the pressure, volume, gas. The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. Ap chem guide's crash course on the ideal gas law. Gas laws summary ap chemistry summary #1 1. To find the moles,. Ap Chemistry Lab Gas Laws.
From arbronsipva.weebly.com
Gaslawlabsforapchemistry REPACK Ap Chemistry Lab Gas Laws Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Gas laws summary ap chemistry summary #1 1. Using the data provided through the procedures, the numerical value of the pressure, volume, gas. Ap chem guide's crash course on the ideal gas law. To find the moles, it was essential to utilize. Ap Chemistry Lab Gas Laws.
From www.youtube.com
AP Chem Ch 10 Gas Laws Problems YouTube Ap Chemistry Lab Gas Laws Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Gas laws summary. Ap Chemistry Lab Gas Laws.
From studylib.net
AP Chemistry Gas Laws and Stoichiometry 3 Scenarios Ap Chemistry Lab Gas Laws The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. Ap chem guide's crash course on the ideal gas law. Using the data provided through the procedures, the numerical value of the pressure, volume, gas. Use the rearranged ideal gas law given in the background to determine. Ap Chemistry Lab Gas Laws.
From apchemistrygas.weebly.com
Important Laws to Remember! AP Chemistry Term Review Gas Ap Chemistry Lab Gas Laws Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. Ap chem guide's crash course on the ideal gas law. Gas laws summary ap chemistry summary #1 1. The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of. Ap Chemistry Lab Gas Laws.
From studylib.net
Gas Laws Practice Test.Ans.Key Ap Chemistry Lab Gas Laws The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. Gas laws summary ap chemistry summary #1 1. Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. Boyle’s law describes the inverse proportional. Ap Chemistry Lab Gas Laws.
From www.youtube.com
AP Chemistry Gas Density YouTube Ap Chemistry Lab Gas Laws Using the data provided through the procedures, the numerical value of the pressure, volume, gas. Ap chem guide's crash course on the ideal gas law. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). Gas laws summary ap chemistry summary #1 1. Boyle’s law describes the inverse proportional relationship between pressure and volume. Ap Chemistry Lab Gas Laws.
From www.studypool.com
SOLUTION Lab 12 Gas Laws Studypool Ap Chemistry Lab Gas Laws Gas laws summary ap chemistry summary #1 1. The equations that express the relationships among t (temperature), p (pressure), v (volume), and n (number of moles of gas) are known as. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). Use the rearranged ideal gas law given in the background to determine the. Ap Chemistry Lab Gas Laws.
From www.youtube.com
Gas Law Stoichiometry YouTube Ap Chemistry Lab Gas Laws Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. Ap chem guide's crash course on the ideal gas law. Gas laws summary ap chemistry summary #1 1. Boyle’s law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed. Using. Ap Chemistry Lab Gas Laws.
From www.studocu.com
AP Chemistry Unit 3 Gases SC 32 Answers Unit 3 Properties of Gases Ap Chemistry Lab Gas Laws Using the data provided through the procedures, the numerical value of the pressure, volume, gas. Use the rearranged ideal gas law given in the background to determine the molar mass (m) in g/mol of butane for each trial. To find the moles, it was essential to utilize the ideal gas law (pv = nrt). Gas laws summary ap chemistry summary. Ap Chemistry Lab Gas Laws.