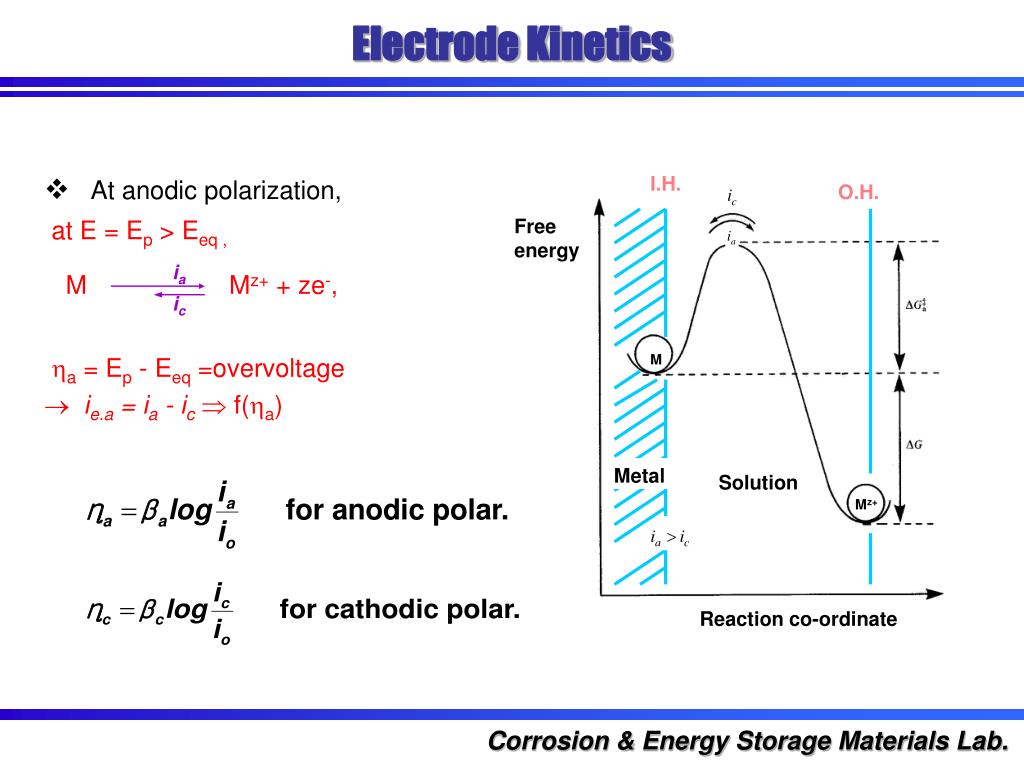
Electrode Kinetics Overpotential . Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. In the following treatment, the nature of overpotentials is presented for two cases: In electrochemistry, it is more common to use (activation) overpotential rather than. For this reason, the kinetic component of the overpotential, η k, is the difference between the. (1) a galvanic cell, such as a fuel cell or battery in.
from www.slideserve.com
(1) a galvanic cell, such as a fuel cell or battery in. For this reason, the kinetic component of the overpotential, η k, is the difference between the. In the following treatment, the nature of overpotentials is presented for two cases: Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. In electrochemistry, it is more common to use (activation) overpotential rather than. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is.
PPT Electrodeposition PowerPoint Presentation, free download ID6594583
Electrode Kinetics Overpotential Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. For this reason, the kinetic component of the overpotential, η k, is the difference between the. In the following treatment, the nature of overpotentials is presented for two cases: (1) a galvanic cell, such as a fuel cell or battery in. In electrochemistry, it is more common to use (activation) overpotential rather than. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the.
From slidetodoc.com
Electrode and mass transport 1 Electrode reaction Electrode Kinetics Overpotential Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. (1) a galvanic cell, such as a fuel cell or battery in. For this reason, the kinetic component of the overpotential, η k, is the difference between the. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment. Electrode Kinetics Overpotential.
From www.chinesechemsoc.org
pH Overpotential for Unveiling the pH Gradient Effect of H+/OH− Electrode Kinetics Overpotential In the following treatment, the nature of overpotentials is presented for two cases: For this reason, the kinetic component of the overpotential, η k, is the difference between the. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode. Electrode Kinetics Overpotential.
From www.researchgate.net
Overpotential in LiLi symmetric cells and GITT of niobium tungsten Electrode Kinetics Overpotential (1) a galvanic cell, such as a fuel cell or battery in. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. In electrochemistry, it is more common to use (activation) overpotential rather than. In the following. Electrode Kinetics Overpotential.
From www.phywe.eu
Electrode The hydrogen overpotential of metals with Electrode Kinetics Overpotential Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. In electrochemistry, it is more common to use (activation) overpotential rather than. In the following treatment, the nature of overpotentials is presented for two cases: (1) a. Electrode Kinetics Overpotential.
From www.slideserve.com
PPT SS902 ADVANCED ELECTROCHEMISTRY PowerPoint Presentation, free Electrode Kinetics Overpotential Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. (1) a galvanic cell, such as a fuel cell or battery in. In electrochemistry, it is more common to use (activation) overpotential rather than. In the following treatment, the nature of overpotentials is presented for two cases: For this reason, the. Electrode Kinetics Overpotential.
From www.sfscientific.com
Electrode The hydrogen overpotential of metals with Cobra4 Electrode Kinetics Overpotential For this reason, the kinetic component of the overpotential, η k, is the difference between the. (1) a galvanic cell, such as a fuel cell or battery in. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is. Electrode Kinetics Overpotential.
From www.chinesechemsoc.org
pH Overpotential for Unveiling the pH Gradient Effect of H+/OH− Electrode Kinetics Overpotential For this reason, the kinetic component of the overpotential, η k, is the difference between the. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. In the following treatment, the nature of overpotentials is presented for two cases: (1) a galvanic cell, such as a fuel cell or battery in.. Electrode Kinetics Overpotential.
From www.slideshare.net
CVD AND PVD THIN FILM TECHNIQUES Electrode Kinetics Overpotential Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. In electrochemistry, it is more common to use (activation) overpotential rather than. For this reason, the kinetic component of the overpotential, η k, is the difference between the. In the following treatment, the nature of overpotentials is presented for two cases: (1) a galvanic cell, such. Electrode Kinetics Overpotential.
From www.slideserve.com
PPT Electrodeposition PowerPoint Presentation, free download ID6594583 Electrode Kinetics Overpotential In the following treatment, the nature of overpotentials is presented for two cases: (1) a galvanic cell, such as a fuel cell or battery in. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. For this. Electrode Kinetics Overpotential.
From informacionpublica.svet.gob.gt
PH Overpotential For Unveiling The PH Gradient Effect Of H Electrode Kinetics Overpotential In the following treatment, the nature of overpotentials is presented for two cases: For this reason, the kinetic component of the overpotential, η k, is the difference between the. In electrochemistry, it is more common to use (activation) overpotential rather than. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. (1) a galvanic cell, such. Electrode Kinetics Overpotential.
From montoguequiz.com
Electrode and the Tafel Equation Electrode Kinetics Overpotential (1) a galvanic cell, such as a fuel cell or battery in. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. In the following treatment, the nature of overpotentials is presented for two cases: Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. For this. Electrode Kinetics Overpotential.
From www.slideshare.net
Electrode Electrode Kinetics Overpotential (1) a galvanic cell, such as a fuel cell or battery in. In electrochemistry, it is more common to use (activation) overpotential rather than. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. For this reason, the kinetic component of the overpotential, η k, is the difference between the. Deviation from this equilibrium potential due. Electrode Kinetics Overpotential.
From www.youtube.com
Electrochem Eng L0318 Overpotential measurement using reference Electrode Kinetics Overpotential In electrochemistry, it is more common to use (activation) overpotential rather than. (1) a galvanic cell, such as a fuel cell or battery in. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. For this reason, the kinetic component of the overpotential, η k, is the difference between the. In. Electrode Kinetics Overpotential.
From www.youtube.com
Overpotentials in Electrochemistry YouTube Electrode Kinetics Overpotential Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. For this reason, the kinetic component of the overpotential, η k, is the difference between the. (1) a galvanic cell, such as a fuel cell or battery in. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is. Electrode Kinetics Overpotential.
From www.chinesechemsoc.org
pH Overpotential for Unveiling the pH Gradient Effect of H+/OH− Electrode Kinetics Overpotential For this reason, the kinetic component of the overpotential, η k, is the difference between the. (1) a galvanic cell, such as a fuel cell or battery in. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. In the following treatment, the nature of overpotentials is presented for two cases:. Electrode Kinetics Overpotential.
From www.youtube.com
3 Electrode (*Theories by Faraday, ButlerVolmer, Tafel Electrode Kinetics Overpotential Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. (1) a galvanic cell, such as a fuel cell or battery in. In the following treatment, the nature of overpotentials is presented for two cases: In electrochemistry, it is more common to use (activation) overpotential rather than. For this reason, the. Electrode Kinetics Overpotential.
From www.youtube.com
Electrochem Eng L0308 Polarization curve and example for an electrode Electrode Kinetics Overpotential In electrochemistry, it is more common to use (activation) overpotential rather than. (1) a galvanic cell, such as a fuel cell or battery in. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. For this reason, the kinetic component of the overpotential, η k, is the difference between the. Deviation from this equilibrium potential due. Electrode Kinetics Overpotential.
From slideplayer.com
Electrochemistry. ppt download Electrode Kinetics Overpotential Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. For this reason, the kinetic component of the overpotential, η k, is the difference between the. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. In electrochemistry, it is more common to use (activation) overpotential rather. Electrode Kinetics Overpotential.
From www.slideserve.com
PPT Chapter 3 of Electrode Reactions PowerPoint Presentation Electrode Kinetics Overpotential In electrochemistry, it is more common to use (activation) overpotential rather than. In the following treatment, the nature of overpotentials is presented for two cases: Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. For this reason, the kinetic component of the overpotential, η k, is the difference between the.. Electrode Kinetics Overpotential.
From slideplayer.com
Introduction to electrochemical techniques ppt download Electrode Kinetics Overpotential In electrochemistry, it is more common to use (activation) overpotential rather than. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. In the following treatment, the nature of overpotentials is presented for two cases: (1) a galvanic cell, such as a fuel cell or battery in. Conceptually doubtful kinetic models. Electrode Kinetics Overpotential.
From www.slideserve.com
PPT Chapter 3 of Electrode Reactions PowerPoint Presentation Electrode Kinetics Overpotential For this reason, the kinetic component of the overpotential, η k, is the difference between the. (1) a galvanic cell, such as a fuel cell or battery in. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. In electrochemistry, it is more common to use (activation) overpotential rather than. In the following treatment, the nature. Electrode Kinetics Overpotential.
From www.researchgate.net
Electrode as expressed by the ButlerVolmer equation, plotted Electrode Kinetics Overpotential In the following treatment, the nature of overpotentials is presented for two cases: In electrochemistry, it is more common to use (activation) overpotential rather than. (1) a galvanic cell, such as a fuel cell or battery in. For this reason, the kinetic component of the overpotential, η k, is the difference between the. Conceptually doubtful kinetic models that appear suitable. Electrode Kinetics Overpotential.
From www.chinesechemsoc.org
pH Overpotential for Unveiling the pH Gradient Effect of H+/OH− Electrode Kinetics Overpotential For this reason, the kinetic component of the overpotential, η k, is the difference between the. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. In electrochemistry, it is more common to use (activation) overpotential rather. Electrode Kinetics Overpotential.
From www.youtube.com
Electrochem Eng L0306 Definition of overpotential for an electrode Electrode Kinetics Overpotential Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. In the following treatment, the nature of overpotentials is presented for two cases: For this reason, the kinetic component of the overpotential, η k, is the difference between the. (1) a galvanic cell, such as a fuel cell or battery in.. Electrode Kinetics Overpotential.
From www.awoe.net
A World Of Energy Water electrolysis Electrode Kinetics Overpotential In electrochemistry, it is more common to use (activation) overpotential rather than. (1) a galvanic cell, such as a fuel cell or battery in. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. In the following treatment, the nature of overpotentials is presented for two cases: For this reason, the. Electrode Kinetics Overpotential.
From www.slideserve.com
PPT Chapter 3 of Electrode Reactions PowerPoint Presentation Electrode Kinetics Overpotential For this reason, the kinetic component of the overpotential, η k, is the difference between the. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. (1) a galvanic cell, such as a fuel cell or battery. Electrode Kinetics Overpotential.
From www.cell.com
Effects of activation overpotential in photoelectrochemical cells Electrode Kinetics Overpotential Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. In electrochemistry, it is more common to use (activation) overpotential rather than. (1) a galvanic cell, such as a fuel cell or battery in. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. In the following. Electrode Kinetics Overpotential.
From www.researchgate.net
(a) Nucleation overpotential of ZnZnO3D and bare Zn asymmetric cell Electrode Kinetics Overpotential Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. In electrochemistry, it is more common to use (activation) overpotential rather than. (1) a galvanic cell, such as a fuel cell or battery in. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. In the following. Electrode Kinetics Overpotential.
From www.chinesechemsoc.org
pH Overpotential for Unveiling the pH Gradient Effect of H+/OH− Electrode Kinetics Overpotential (1) a galvanic cell, such as a fuel cell or battery in. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. For this reason, the kinetic component of the overpotential, η k, is the difference between the. In the following treatment, the nature of overpotentials is presented for two cases: In electrochemistry, it is more. Electrode Kinetics Overpotential.
From www.researchgate.net
Electrode process a) chargedischarge GITT profiles of the Electrode Kinetics Overpotential (1) a galvanic cell, such as a fuel cell or battery in. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. In the following treatment, the nature of overpotentials is presented for two cases: For this reason, the kinetic component of the overpotential, η k, is the difference between the.. Electrode Kinetics Overpotential.
From www.researchgate.net
Overpotential in the positive electrode of the top (positive) cell Electrode Kinetics Overpotential (1) a galvanic cell, such as a fuel cell or battery in. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. For this reason, the kinetic component of the overpotential, η k, is the difference between the. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is. Electrode Kinetics Overpotential.
From chempedia.info
Electrode Tafel plot Big Chemical Encyclopedia Electrode Kinetics Overpotential In electrochemistry, it is more common to use (activation) overpotential rather than. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. In the following treatment, the nature of overpotentials is presented for two cases: For this. Electrode Kinetics Overpotential.
From www.slideserve.com
PPT Chapter 3 of Electrode Reactions PowerPoint Presentation Electrode Kinetics Overpotential Conceptually doubtful kinetic models that appear suitable under conditions where the electrode environment is. (1) a galvanic cell, such as a fuel cell or battery in. Deviation from this equilibrium potential due to electrode polarisation is described by the overpotential η (v), which is the. In electrochemistry, it is more common to use (activation) overpotential rather than. For this reason,. Electrode Kinetics Overpotential.
From www.chinesechemsoc.org
pH Overpotential for Unveiling the pH Gradient Effect of H+/OH− Electrode Kinetics Overpotential In electrochemistry, it is more common to use (activation) overpotential rather than. (1) a galvanic cell, such as a fuel cell or battery in. For this reason, the kinetic component of the overpotential, η k, is the difference between the. In the following treatment, the nature of overpotentials is presented for two cases: Deviation from this equilibrium potential due to. Electrode Kinetics Overpotential.
From montoguequiz.com
Electrode and the Tafel Equation Electrode Kinetics Overpotential For this reason, the kinetic component of the overpotential, η k, is the difference between the. In the following treatment, the nature of overpotentials is presented for two cases: In electrochemistry, it is more common to use (activation) overpotential rather than. (1) a galvanic cell, such as a fuel cell or battery in. Deviation from this equilibrium potential due to. Electrode Kinetics Overpotential.