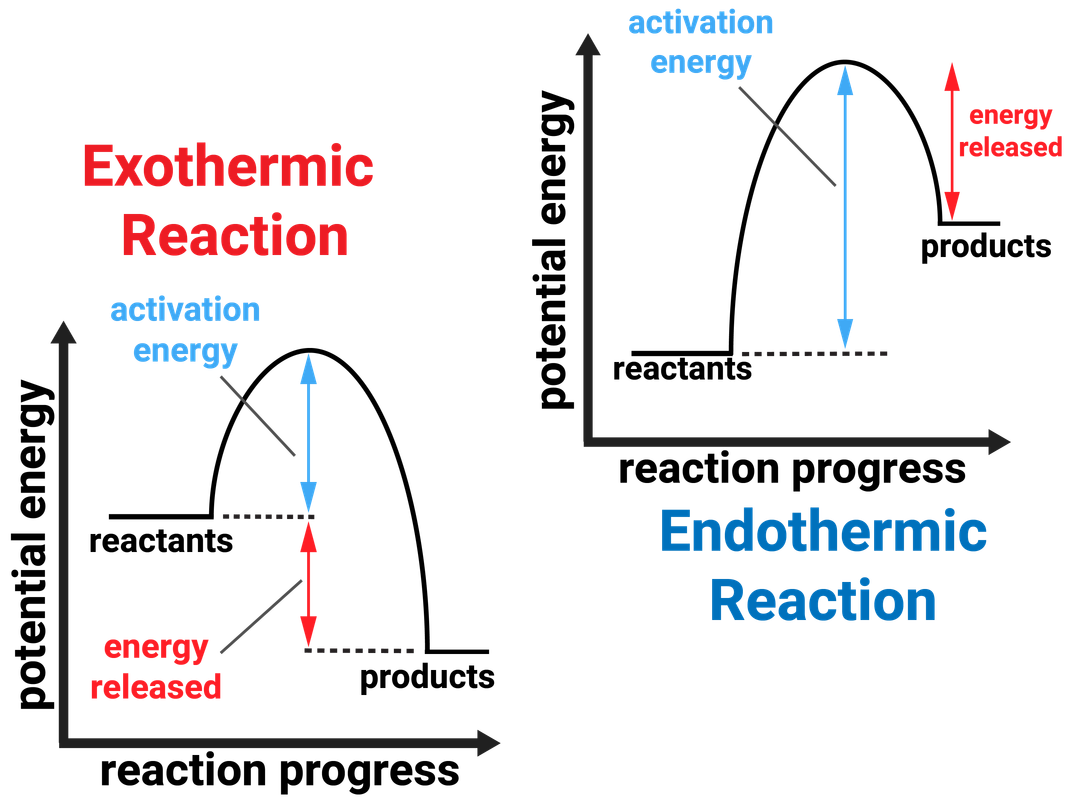
Endothermic Activation Energy Change . This may be a change in heat, electricity, light, or other forms of energy. Exothermic reactions give out energy and the temperature increases. The energy level increases in an endothermic reaction. The transfer of thermal energy during a reaction is called the enthalpy change, δh, of the reaction. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Energy changes because bonds rearrange to make new bonds with different energies. Chemical reactions involve changes in energy. Reactions with higher activation energies require more energy to start than those with lower activation energies. Δh is a positive value for endothermic reactions. This is because energy is taken in from the surroundings. Energy changes in chemical reactions activation energy. Reaction a is exothermic because heat is leaving the system. An upwards arrow shows that.
from revisechemistry.uk
Reaction a is exothermic because heat is leaving the system. An upwards arrow shows that. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. This may be a change in heat, electricity, light, or other forms of energy. Exothermic reactions give out energy and the temperature increases. Energy changes in chemical reactions activation energy. This is because energy is taken in from the surroundings. Δh is a positive value for endothermic reactions. Energy changes because bonds rearrange to make new bonds with different energies. The transfer of thermal energy during a reaction is called the enthalpy change, δh, of the reaction.
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk
Endothermic Activation Energy Change An upwards arrow shows that. This may be a change in heat, electricity, light, or other forms of energy. Chemical reactions involve changes in energy. Reaction a is exothermic because heat is leaving the system. The energy level increases in an endothermic reaction. Reactions with higher activation energies require more energy to start than those with lower activation energies. An upwards arrow shows that. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Energy changes because bonds rearrange to make new bonds with different energies. This is because energy is taken in from the surroundings. The transfer of thermal energy during a reaction is called the enthalpy change, δh, of the reaction. Exothermic reactions give out energy and the temperature increases. Energy changes in chemical reactions activation energy. Δh is a positive value for endothermic reactions.
From www.vrogue.co
Draw The Enthalpy Diagram For Exothermic And Endother vrogue.co Endothermic Activation Energy Change Δh is a positive value for endothermic reactions. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. The transfer of thermal energy during a reaction is called the enthalpy change, δh, of the reaction. Chemical reactions involve changes in energy. Energy changes in chemical reactions activation energy. Exothermic reactions. Endothermic Activation Energy Change.
From slideplayer.com
Exothermic & Endothermic Reactions Energy Diagrams ppt download Endothermic Activation Energy Change An upwards arrow shows that. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Reactions with higher activation energies require more energy to start than those with lower activation energies. Energy changes in chemical reactions activation energy. The energy level increases in an endothermic reaction. Energy changes because bonds. Endothermic Activation Energy Change.
From stock.adobe.com
activation energy endothermic reaction diagram vector de Stock Adobe Endothermic Activation Energy Change Reactions with higher activation energies require more energy to start than those with lower activation energies. The transfer of thermal energy during a reaction is called the enthalpy change, δh, of the reaction. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Reaction a is exothermic because heat is. Endothermic Activation Energy Change.
From slideplayer.com
Exothermic & Endothermic Reactions Energy Diagrams ppt download Endothermic Activation Energy Change Δh is a positive value for endothermic reactions. Reactions with higher activation energies require more energy to start than those with lower activation energies. This is because energy is taken in from the surroundings. Reaction a is exothermic because heat is leaving the system. An upwards arrow shows that. Energy changes in chemical reactions activation energy. The transfer of thermal. Endothermic Activation Energy Change.
From schematicdbelfriede.z13.web.core.windows.net
Energy Diagram For Endothermic Reaction Endothermic Activation Energy Change This is because energy is taken in from the surroundings. Δh is a positive value for endothermic reactions. Chemical reactions involve changes in energy. Reaction a is exothermic because heat is leaving the system. This may be a change in heat, electricity, light, or other forms of energy. Exothermic reactions give out energy and the temperature increases. Energy changes in. Endothermic Activation Energy Change.
From wiringdatabaseinfo.blogspot.com
Energy Diagram For Endothermic Reaction Wiring Site Resource Endothermic Activation Energy Change Energy changes because bonds rearrange to make new bonds with different energies. Chemical reactions involve changes in energy. This may be a change in heat, electricity, light, or other forms of energy. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Δh is a positive value for endothermic reactions.. Endothermic Activation Energy Change.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Endothermic Activation Energy Change Exothermic reactions give out energy and the temperature increases. Δh is a positive value for endothermic reactions. Energy changes in chemical reactions activation energy. Energy changes because bonds rearrange to make new bonds with different energies. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. The transfer of thermal. Endothermic Activation Energy Change.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Activation Energy Change Δh is a positive value for endothermic reactions. The energy level increases in an endothermic reaction. This is because energy is taken in from the surroundings. Reactions with higher activation energies require more energy to start than those with lower activation energies. Exothermic reactions give out energy and the temperature increases. The changes in energy that occur during a chemical. Endothermic Activation Energy Change.
From www.istockphoto.com
Vector Graphs Of Endothermic And Exothermic Reactions Activation Energy Endothermic Activation Energy Change The transfer of thermal energy during a reaction is called the enthalpy change, δh, of the reaction. Exothermic reactions give out energy and the temperature increases. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. An upwards arrow shows that. This may be a change in heat, electricity, light,. Endothermic Activation Energy Change.
From www.expii.com
Energy Diagram — Overview & Parts Expii Endothermic Activation Energy Change Energy changes in chemical reactions activation energy. Reaction a is exothermic because heat is leaving the system. This may be a change in heat, electricity, light, or other forms of energy. Δh is a positive value for endothermic reactions. This is because energy is taken in from the surroundings. The changes in energy that occur during a chemical reaction can. Endothermic Activation Energy Change.
From elecdiags.com
The Significance and Use of Endothermic Reaction Profile Diagrams in Endothermic Activation Energy Change Energy changes in chemical reactions activation energy. The energy level increases in an endothermic reaction. Reaction a is exothermic because heat is leaving the system. Chemical reactions involve changes in energy. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Reactions with higher activation energies require more energy to. Endothermic Activation Energy Change.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Endothermic Activation Energy Change Chemical reactions involve changes in energy. Δh is a positive value for endothermic reactions. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Reactions with higher activation energies require more energy to start than those with lower activation energies. This may be a change in heat, electricity, light, or. Endothermic Activation Energy Change.
From studylib.net
Lesson 7 Reaction profiles Endothermic Activation Energy Change Chemical reactions involve changes in energy. Energy changes because bonds rearrange to make new bonds with different energies. Energy changes in chemical reactions activation energy. Reactions with higher activation energies require more energy to start than those with lower activation energies. The transfer of thermal energy during a reaction is called the enthalpy change, δh, of the reaction. Δh is. Endothermic Activation Energy Change.
From www.doubtnut.com
For an endothermic reaction energy of activation is E(a) and enthlpy o Endothermic Activation Energy Change Exothermic reactions give out energy and the temperature increases. Energy changes in chemical reactions activation energy. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. This is because energy is taken in from the surroundings. Chemical reactions involve changes in energy. The transfer of thermal energy during a reaction. Endothermic Activation Energy Change.
From www.savemyexams.co.uk
Enthalpy Change & Activation Energy (5.1.2) CIE IGCSE Chemistry Endothermic Activation Energy Change This is because energy is taken in from the surroundings. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. An upwards arrow shows that. Energy changes in chemical reactions activation energy. Exothermic reactions give out energy and the temperature increases. The energy level increases in an endothermic reaction. Reactions. Endothermic Activation Energy Change.
From www.studypool.com
SOLUTION Energy changes in chemical reactions activation energy Endothermic Activation Energy Change Reaction a is exothermic because heat is leaving the system. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Δh is a positive value for endothermic reactions. This is because energy is taken in from the surroundings. Reactions with higher activation energies require more energy to start than those. Endothermic Activation Energy Change.
From www.youtube.com
Endothermic and Exothermic Reactions With Potential Energy Diagrams Endothermic Activation Energy Change The transfer of thermal energy during a reaction is called the enthalpy change, δh, of the reaction. An upwards arrow shows that. This is because energy is taken in from the surroundings. This may be a change in heat, electricity, light, or other forms of energy. Reactions with higher activation energies require more energy to start than those with lower. Endothermic Activation Energy Change.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID5592422 Endothermic Activation Energy Change This is because energy is taken in from the surroundings. Energy changes because bonds rearrange to make new bonds with different energies. This may be a change in heat, electricity, light, or other forms of energy. The transfer of thermal energy during a reaction is called the enthalpy change, δh, of the reaction. Exothermic reactions give out energy and the. Endothermic Activation Energy Change.
From socratic.org
What are activation energies? Socratic Endothermic Activation Energy Change Reactions with higher activation energies require more energy to start than those with lower activation energies. This may be a change in heat, electricity, light, or other forms of energy. Exothermic reactions give out energy and the temperature increases. This is because energy is taken in from the surroundings. Δh is a positive value for endothermic reactions. The energy level. Endothermic Activation Energy Change.
From chemnotcheem.com
Enthalpy change of reactions O Level Chemistry Notes Endothermic Activation Energy Change The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Reaction a is exothermic because heat is leaving the system. Exothermic reactions give out energy and the temperature increases. The energy level increases in an endothermic reaction. The transfer of thermal energy during a reaction is called the enthalpy change,. Endothermic Activation Energy Change.
From www.youtube.com
How to draw Energy Profile Diagram and Energy Level Diagram of Endothermic Activation Energy Change Δh is a positive value for endothermic reactions. Chemical reactions involve changes in energy. Reactions with higher activation energies require more energy to start than those with lower activation energies. The transfer of thermal energy during a reaction is called the enthalpy change, δh, of the reaction. Energy changes because bonds rearrange to make new bonds with different energies. This. Endothermic Activation Energy Change.
From byjus.com
24. What is the Minimum activation energy required for endothermic and Endothermic Activation Energy Change The transfer of thermal energy during a reaction is called the enthalpy change, δh, of the reaction. Reactions with higher activation energies require more energy to start than those with lower activation energies. Exothermic reactions give out energy and the temperature increases. Δh is a positive value for endothermic reactions. The changes in energy that occur during a chemical reaction. Endothermic Activation Energy Change.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Activation Energy Change Reactions with higher activation energies require more energy to start than those with lower activation energies. The energy level increases in an endothermic reaction. Energy changes in chemical reactions activation energy. Chemical reactions involve changes in energy. Δh is a positive value for endothermic reactions. The changes in energy that occur during a chemical reaction can be seen by examining. Endothermic Activation Energy Change.
From telegra.ph
Endothermic energy profile diagram activation Telegraph Endothermic Activation Energy Change An upwards arrow shows that. Chemical reactions involve changes in energy. Δh is a positive value for endothermic reactions. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Energy changes because bonds rearrange to make new bonds with different energies. This is because energy is taken in from the. Endothermic Activation Energy Change.
From manuallistcantabank.z21.web.core.windows.net
Endothermic Energy Diagram Endothermic Activation Energy Change Δh is a positive value for endothermic reactions. Reaction a is exothermic because heat is leaving the system. An upwards arrow shows that. The energy level increases in an endothermic reaction. This may be a change in heat, electricity, light, or other forms of energy. Reactions with higher activation energies require more energy to start than those with lower activation. Endothermic Activation Energy Change.
From jumpstarterdiscount.blogspot.com
Energy Diagram For Endothermic Reaction Wiring Diagram Endothermic Activation Energy Change The transfer of thermal energy during a reaction is called the enthalpy change, δh, of the reaction. Reactions with higher activation energies require more energy to start than those with lower activation energies. The energy level increases in an endothermic reaction. This may be a change in heat, electricity, light, or other forms of energy. This is because energy is. Endothermic Activation Energy Change.
From www.studypool.com
SOLUTION Energy changes in chemical reactions activation energy Endothermic Activation Energy Change Δh is a positive value for endothermic reactions. Energy changes because bonds rearrange to make new bonds with different energies. Reaction a is exothermic because heat is leaving the system. This may be a change in heat, electricity, light, or other forms of energy. This is because energy is taken in from the surroundings. The transfer of thermal energy during. Endothermic Activation Energy Change.
From professional.patrickneyman.com
Activation Energy Equation Endothermic Activation Energy Change The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. An upwards arrow shows that. Exothermic reactions give out energy and the temperature increases. Reaction a is exothermic because heat is leaving the system. Energy changes in chemical reactions activation energy. The energy level increases in an endothermic reaction. Energy. Endothermic Activation Energy Change.
From elecdiags.com
The Significance and Use of Endothermic Reaction Profile Diagrams in Endothermic Activation Energy Change The energy level increases in an endothermic reaction. Reaction a is exothermic because heat is leaving the system. Reactions with higher activation energies require more energy to start than those with lower activation energies. Energy changes in chemical reactions activation energy. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical. Endothermic Activation Energy Change.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Activation Energy Change An upwards arrow shows that. The transfer of thermal energy during a reaction is called the enthalpy change, δh, of the reaction. Chemical reactions involve changes in energy. Exothermic reactions give out energy and the temperature increases. The energy level increases in an endothermic reaction. Energy changes because bonds rearrange to make new bonds with different energies. Reactions with higher. Endothermic Activation Energy Change.
From www.dreamstime.com
Activation Energy Endothermic Reaction 2d Illustration Stock Endothermic Activation Energy Change An upwards arrow shows that. This may be a change in heat, electricity, light, or other forms of energy. This is because energy is taken in from the surroundings. Δh is a positive value for endothermic reactions. Chemical reactions involve changes in energy. The transfer of thermal energy during a reaction is called the enthalpy change, δh, of the reaction.. Endothermic Activation Energy Change.
From www.slideserve.com
PPT Energy Changes PowerPoint Presentation, free download ID3470966 Endothermic Activation Energy Change Δh is a positive value for endothermic reactions. This may be a change in heat, electricity, light, or other forms of energy. This is because energy is taken in from the surroundings. The energy level increases in an endothermic reaction. Chemical reactions involve changes in energy. Reaction a is exothermic because heat is leaving the system. Exothermic reactions give out. Endothermic Activation Energy Change.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Activation Energy Change Reaction a is exothermic because heat is leaving the system. Δh is a positive value for endothermic reactions. This may be a change in heat, electricity, light, or other forms of energy. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. This is because energy is taken in from. Endothermic Activation Energy Change.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation ID239015 Endothermic Activation Energy Change Δh is a positive value for endothermic reactions. Energy changes because bonds rearrange to make new bonds with different energies. An upwards arrow shows that. Chemical reactions involve changes in energy. Exothermic reactions give out energy and the temperature increases. Reaction a is exothermic because heat is leaving the system. The energy level increases in an endothermic reaction. Energy changes. Endothermic Activation Energy Change.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Activation Energy Change This may be a change in heat, electricity, light, or other forms of energy. An upwards arrow shows that. Chemical reactions involve changes in energy. Exothermic reactions give out energy and the temperature increases. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. The transfer of thermal energy during. Endothermic Activation Energy Change.