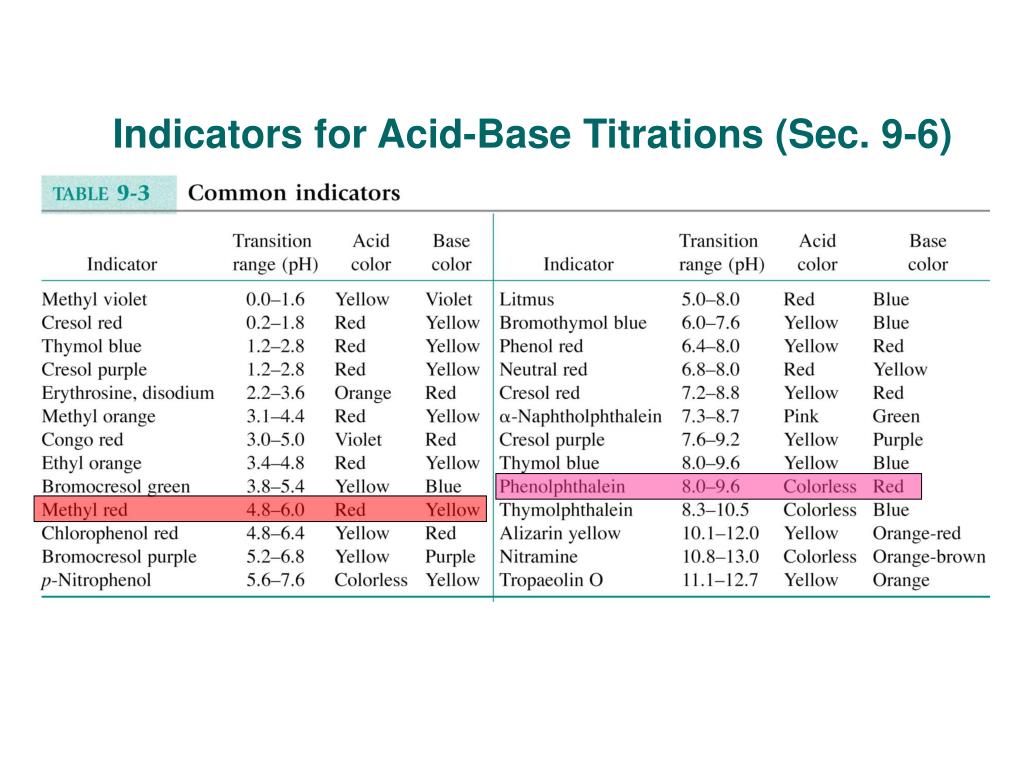
Indicator For Base Titration . Calculating ph for titration solutions: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. The graph shows the results obtained using two. You obviously need to choose. In its simplest form, titration is carried out by. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions.
from www.slideserve.com
You obviously need to choose. The graph shows the results obtained using two. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In its simplest form, titration is carried out by. Calculating ph for titration solutions:
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint
Indicator For Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In its simplest form, titration is carried out by. Calculating ph for titration solutions: The graph shows the results obtained using two. You obviously need to choose. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Indicator For Base Titration You obviously need to choose. Calculating ph for titration solutions: The graph shows the results obtained using two. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. In its simplest form, titration is carried. Indicator For Base Titration.
From giorxivqj.blob.core.windows.net
Best Indicator For Hcl And Naoh Titration at Justin Leon blog Indicator For Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In its simplest form, titration is carried out by. You obviously need to choose. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. The graph shows the results obtained using two. Calculating ph for. Indicator For Base Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicator For Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Calculating ph for titration solutions: Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. In its simplest form, titration is carried out by. You obviously need to choose. The graph shows the results obtained. Indicator For Base Titration.
From mmerevise.co.uk
pH Curves Questions and Revision MME Indicator For Base Titration In its simplest form, titration is carried out by. The graph shows the results obtained using two. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Calculating ph for titration solutions: You obviously need. Indicator For Base Titration.
From giorvlvoc.blob.core.windows.net
Acid Base Titration Curve at Adrian Yount blog Indicator For Base Titration Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. Calculating ph for titration solutions: In its simplest form, titration is carried out by. The graph shows the results obtained using two. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. You obviously need. Indicator For Base Titration.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Indicator For Base Titration The graph shows the results obtained using two. In its simplest form, titration is carried out by. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Calculating ph for titration solutions: Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. You obviously need. Indicator For Base Titration.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts Indicator For Base Titration Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. The graph shows the results obtained using two. You obviously need to choose. Calculating ph for titration solutions: In its simplest form, titration is carried out by. A titration is carried out for 25.00 ml of 0.100 m hcl (strong. Indicator For Base Titration.
From mungfali.com
Acid Base Titration Graph Indicator For Base Titration Calculating ph for titration solutions: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In its simplest form, titration is carried out by. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. You obviously need to choose. The graph shows the results obtained. Indicator For Base Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicator For Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. The graph shows the results obtained using two. In its simplest form, titration is carried out by. Calculating ph for titration solutions: Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. You obviously need. Indicator For Base Titration.
From giorxivqj.blob.core.windows.net
Best Indicator For Hcl And Naoh Titration at Justin Leon blog Indicator For Base Titration The graph shows the results obtained using two. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. In its simplest form, titration is carried out by. You obviously need to choose. Calculating ph for. Indicator For Base Titration.
From giodaqksz.blob.core.windows.net
Acid Base Titration Guide at Mary Mcgee blog Indicator For Base Titration You obviously need to choose. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. The graph shows the results obtained using two. Calculating ph for titration solutions: In its simplest form, titration is carried out by. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. Indicator For Base Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Indicator For Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. You obviously need to choose. Calculating ph for titration solutions: The graph shows the results obtained using two. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. In its simplest form, titration is carried. Indicator For Base Titration.
From mungfali.com
Acid Base Titration Indicator Indicator For Base Titration The graph shows the results obtained using two. In its simplest form, titration is carried out by. You obviously need to choose. Calculating ph for titration solutions: Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. A titration is carried out for 25.00 ml of 0.100 m hcl (strong. Indicator For Base Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicator For Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. The graph shows the results obtained using two. You obviously need to choose. In its simplest form, titration is carried out by. Calculating ph for titration solutions: Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. Indicator For Base Titration.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Indicator For Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. You obviously need to choose. In its simplest form, titration is carried out by. Calculating ph for titration solutions: Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. The graph shows the results obtained. Indicator For Base Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Indicator For Base Titration Calculating ph for titration solutions: In its simplest form, titration is carried out by. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. The graph shows the results obtained using two. You obviously need to choose. A titration is carried out for 25.00 ml of 0.100 m hcl (strong. Indicator For Base Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Indicator For Base Titration You obviously need to choose. Calculating ph for titration solutions: Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. In its simplest form, titration is carried out by. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. The graph shows the results obtained. Indicator For Base Titration.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Indicator For Base Titration The graph shows the results obtained using two. In its simplest form, titration is carried out by. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Calculating ph for titration solutions: You obviously need. Indicator For Base Titration.
From courses.lumenlearning.com
AcidBase Titrations Boundless Chemistry Indicator For Base Titration Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. Calculating ph for titration solutions: In its simplest form, titration is carried out by. You obviously need to choose. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. The graph shows the results obtained. Indicator For Base Titration.
From www.alamy.com
Phenolphthalein is used as a single indicator in acidbase titrations Indicator For Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. In its simplest form, titration is carried out by. Calculating ph for titration solutions: The graph shows the results obtained using two. You obviously need. Indicator For Base Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicator For Base Titration The graph shows the results obtained using two. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Calculating ph for titration solutions: In its simplest form, titration is carried out by. You obviously need. Indicator For Base Titration.
From mungfali.com
Acid Base Titration Indicator Indicator For Base Titration In its simplest form, titration is carried out by. The graph shows the results obtained using two. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. You obviously need to choose. Calculating ph for titration solutions: A titration is carried out for 25.00 ml of 0.100 m hcl (strong. Indicator For Base Titration.
From worksheets.clipart-library.com
Acid and Bases, Titration, pH Indicators Worksheets (3 Worksheets Indicator For Base Titration You obviously need to choose. The graph shows the results obtained using two. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. In its simplest form, titration is carried out by. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Calculating ph for. Indicator For Base Titration.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Indicator For Base Titration In its simplest form, titration is carried out by. The graph shows the results obtained using two. Calculating ph for titration solutions: You obviously need to choose. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. Indicator For Base Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Indicator For Base Titration Calculating ph for titration solutions: You obviously need to choose. In its simplest form, titration is carried out by. The graph shows the results obtained using two. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. Indicator For Base Titration.
From www.sliderbase.com
Titration Indicator For Base Titration In its simplest form, titration is carried out by. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Calculating ph for titration solutions: Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. The graph shows the results obtained using two. You obviously need. Indicator For Base Titration.
From hxedyezjh.blob.core.windows.net
Acid Base Indicators Titration at Julie Corson blog Indicator For Base Titration In its simplest form, titration is carried out by. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. You obviously need to choose. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. The graph shows the results obtained using two. Calculating ph for. Indicator For Base Titration.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations* — the science sauce Indicator For Base Titration In its simplest form, titration is carried out by. You obviously need to choose. Calculating ph for titration solutions: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. The graph shows the results obtained. Indicator For Base Titration.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Indicator For Base Titration In its simplest form, titration is carried out by. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. The graph shows the results obtained using two. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. Calculating ph for titration solutions: You obviously need. Indicator For Base Titration.
From schoolbag.info
Figure 10.11. Strong Acid and Weak Base Titration Curve A strong acid Indicator For Base Titration The graph shows the results obtained using two. You obviously need to choose. Calculating ph for titration solutions: Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In its simplest form, titration is carried. Indicator For Base Titration.
From www.slideserve.com
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint Indicator For Base Titration The graph shows the results obtained using two. Calculating ph for titration solutions: In its simplest form, titration is carried out by. You obviously need to choose. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. A titration is carried out for 25.00 ml of 0.100 m hcl (strong. Indicator For Base Titration.
From www.vrogue.co
Why Is Phenolphthalein A Good Indicator For Acid Base vrogue.co Indicator For Base Titration Calculating ph for titration solutions: You obviously need to choose. The graph shows the results obtained using two. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In its simplest form, titration is carried out by. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. Indicator For Base Titration.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Indicator For Base Titration In its simplest form, titration is carried out by. The graph shows the results obtained using two. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. Calculating ph for titration solutions: You obviously need to choose. A titration is carried out for 25.00 ml of 0.100 m hcl (strong. Indicator For Base Titration.
From webmis.highland.cc.il.us
AcidBase Titrations Indicator For Base Titration You obviously need to choose. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. Calculating ph for titration solutions: The graph shows the results obtained using two. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In its simplest form, titration is carried. Indicator For Base Titration.
From www.youtube.com
Acids and Bases Choosing an Indicator for Titration YouTube Indicator For Base Titration You obviously need to choose. The graph shows the results obtained using two. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly equation proportions. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In its simplest form, titration is carried out by. Calculating ph for. Indicator For Base Titration.