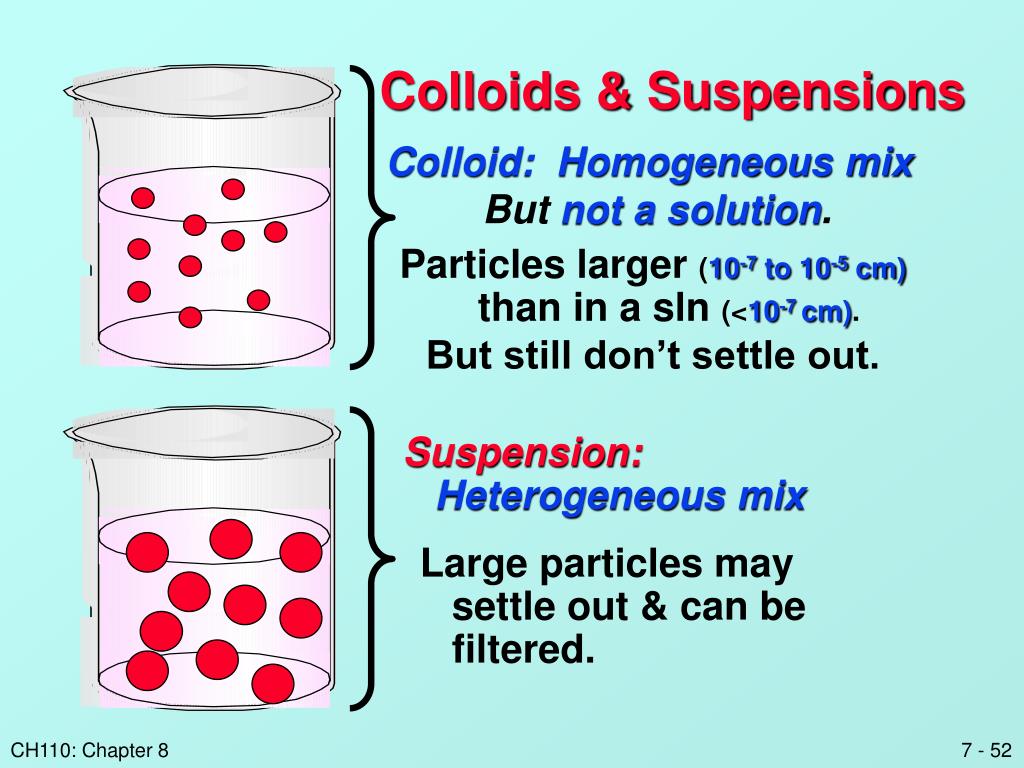
Colloid Solution O . Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. Unlike true solutions where solute. Hydrophilic colloids contain an outer shell of groups that. What is a colloid solution? A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 1). A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. These particles can be solid, liquid, or gas. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. A colloidal solution is a type of mixture which consists of particles whose size varies between 1 and 1000 nanometres. The particles in a colloid are larger. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In a colloidal solution the particles are distributed evenly. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and.
from
A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. A colloidal solution is a type of mixture which consists of particles whose size varies between 1 and 1000 nanometres. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 1). Hydrophilic colloids contain an outer shell of groups that. What is a colloid solution? In a colloidal solution the particles are distributed evenly. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. Unlike true solutions where solute. These particles can be solid, liquid, or gas.
Colloid Solution O A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. A colloidal solution is a type of mixture which consists of particles whose size varies between 1 and 1000 nanometres. Hydrophilic colloids contain an outer shell of groups that. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. In a colloidal solution the particles are distributed evenly. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. These particles can be solid, liquid, or gas. The particles in a colloid are larger. Unlike true solutions where solute. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. What is a colloid solution? A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 1).
From
Colloid Solution O A colloidal solution is a type of mixture which consists of particles whose size varies between 1 and 1000 nanometres. Unlike true solutions where solute. Hydrophilic colloids contain an outer shell of groups that. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. The particles in a colloid are larger. In addition,. Colloid Solution O.
From uen.pressbooks.pub
Colloids Introductory Chemistry Colloid Solution O Unlike true solutions where solute. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. Hydrophilic colloids contain an outer shell of groups that. A colloidal solution is a type of mixture which consists of particles whose size varies between 1 and 1000 nanometres. These particles can. Colloid Solution O.
From wordwall.net
Solution, suspension, colloid Quiz Colloid Solution O The particles in a colloid are larger. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 1). Hydrophilic colloids contain an outer shell of groups that. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. In addition, we will discuss colloids—systems that. Colloid Solution O.
From www.youtube.com
Types of Mixtures Solution, Suspension, Colloid NOTES YouTube Colloid Solution O A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 1). A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. Unlike true solutions where solute. Hydrophilic colloids contain. Colloid Solution O.
From
Colloid Solution O What is a colloid solution? In a colloidal solution the particles are distributed evenly. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. The particles in a colloid are larger. These particles can be solid, liquid, or gas. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles. Colloid Solution O.
From
Colloid Solution O The particles in a colloid are larger. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. These particles can be solid, liquid, or gas. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloids are classified as foams, aerosols,. Colloid Solution O.
From
Colloid Solution O In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. Hydrophilic colloids contain an outer shell of groups that. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. These particles. Colloid Solution O.
From
Colloid Solution O Unlike true solutions where solute. In a colloidal solution the particles are distributed evenly. Hydrophilic colloids contain an outer shell of groups that. The particles in a colloid are larger. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. What is a colloid solution? A group of mixtures called colloids (or colloidal. Colloid Solution O.
From
Colloid Solution O In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. Hydrophilic colloids contain an outer shell of groups that. The particles in a colloid are larger. Unlike true solutions where solute. A colloid can be distinguished from. Colloid Solution O.
From www.slideshare.net
Solutions & colloids Colloid Solution O A colloidal solution is a type of mixture which consists of particles whose size varies between 1 and 1000 nanometres. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. What is a colloid solution? A colloid can be distinguished from a true solution by its ability to scatter. Colloid Solution O.
From www.slideshare.net
Colloids Colloid Solution O Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 1). These particles can be solid, liquid, or gas. A group of mixtures called colloids (or colloidal dispersions) exhibit properties. Colloid Solution O.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Colloid Solution O In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. The particles in a colloid are larger. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. In a colloidal solution the particles are distributed evenly. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending. Colloid Solution O.
From
Colloid Solution O What is a colloid solution? Hydrophilic colloids contain an outer shell of groups that. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. A colloid can be distinguished from a true solution. Colloid Solution O.
From www.slideserve.com
PPT CH110 Chapter 8 Solutions PowerPoint Presentation, free download Colloid Solution O Unlike true solutions where solute. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. A colloidal solution is a type of mixture which consists of particles whose size varies between 1 and 1000 nanometres. A group. Colloid Solution O.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Colloid Solution O A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 1). These particles can be solid, liquid, or gas. A group of mixtures called colloids (or colloidal dispersions). Colloid Solution O.
From ar.inspiredpencil.com
Colloid Suspension Solution Colloid Solution O Hydrophilic colloids contain an outer shell of groups that. The particles in a colloid are larger. These particles can be solid, liquid, or gas. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles. Colloid Solution O.
From www.animalia-life.club
Example Of Colloid Mixture Colloid Solution O Unlike true solutions where solute. These particles can be solid, liquid, or gas. In a colloidal solution the particles are distributed evenly. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. What is a colloid solution? Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed. Colloid Solution O.
From
Colloid Solution O Hydrophilic colloids contain an outer shell of groups that. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. What is a colloid solution? A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Unlike true solutions where solute. Colloids are classified as foams, aerosols, emulsions, gels,. Colloid Solution O.
From
Colloid Solution O In a colloidal solution the particles are distributed evenly. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 1). Unlike true solutions where solute. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. What is a colloid solution?. Colloid Solution O.
From
Colloid Solution O In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. What is a colloid solution? A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions. Colloid Solution O.
From
Colloid Solution O Unlike true solutions where solute. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 1). The particles in a colloid are larger. These particles can be solid, liquid, or gas. In addition, we. Colloid Solution O.
From www.sliderbase.com
Suspensions and Colloids Presentation Chemistry Colloid Solution O A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 1). A colloidal solution is a type of mixture which consists of particles whose size varies between 1 and 1000 nanometres. In a colloidal solution the particles are distributed evenly. A colloidal solution typically consists of particles ranging in size from. Colloid Solution O.
From
Colloid Solution O The particles in a colloid are larger. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. In a colloidal solution the particles are distributed evenly. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending. Colloid Solution O.
From
Colloid Solution O A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. The particles in a colloid are larger. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between. Colloid Solution O.
From
Colloid Solution O Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. The particles in a colloid are larger. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions. Colloid Solution O.
From
Colloid Solution O What is a colloid solution? Unlike true solutions where solute. Hydrophilic colloids contain an outer shell of groups that. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 1). A colloid can be distinguished. Colloid Solution O.
From
Colloid Solution O In a colloidal solution the particles are distributed evenly. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. What is a colloid solution? Unlike true solutions where solute. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. A colloid can be distinguished from a true. Colloid Solution O.
From
Colloid Solution O A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. Unlike true solutions where solute. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 1). A colloidal. Colloid Solution O.
From www.vrogue.co
What Is A Colloid Definition And Examples vrogue.co Colloid Solution O Hydrophilic colloids contain an outer shell of groups that. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 1). A colloidal solution is a type of mixture which consists of particles whose size varies. Colloid Solution O.
From
Colloid Solution O Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. In a colloidal solution the particles are distributed evenly. A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those. Colloid Solution O.
From pt.slideshare.net
Solutions, suspensions, and colloids Colloid Solution O A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and. What is a colloid solution? These particles can be solid, liquid, or gas. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. In a colloidal solution the particles are distributed evenly.. Colloid Solution O.
From 1svoimi-rukami.ru
Как сделать коллоидный раствор дома 82 фото Colloid Solution O In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of the dispersed phase and dispersion medium. In a colloidal solution the particles are distributed evenly. A colloidal solution typically consists of particles ranging in size from 1 nanometer to. Colloid Solution O.
From
Colloid Solution O These particles can be solid, liquid, or gas. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. What is a colloid solution? A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 1). Hydrophilic colloids contain an outer shell of groups that. In addition,. Colloid Solution O.
From
Colloid Solution O A group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions (figure 1). What is a colloid solution? A colloidal solution is a type of mixture which consists of particles whose size varies between 1 and 1000 nanometres. Colloids are classified as foams, aerosols, emulsions, gels, or sols, depending on the nature of. Colloid Solution O.
From
Colloid Solution O What is a colloid solution? A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. A colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. The particles in a colloid are larger. A group of mixtures called colloids (or colloidal dispersions). Colloid Solution O.