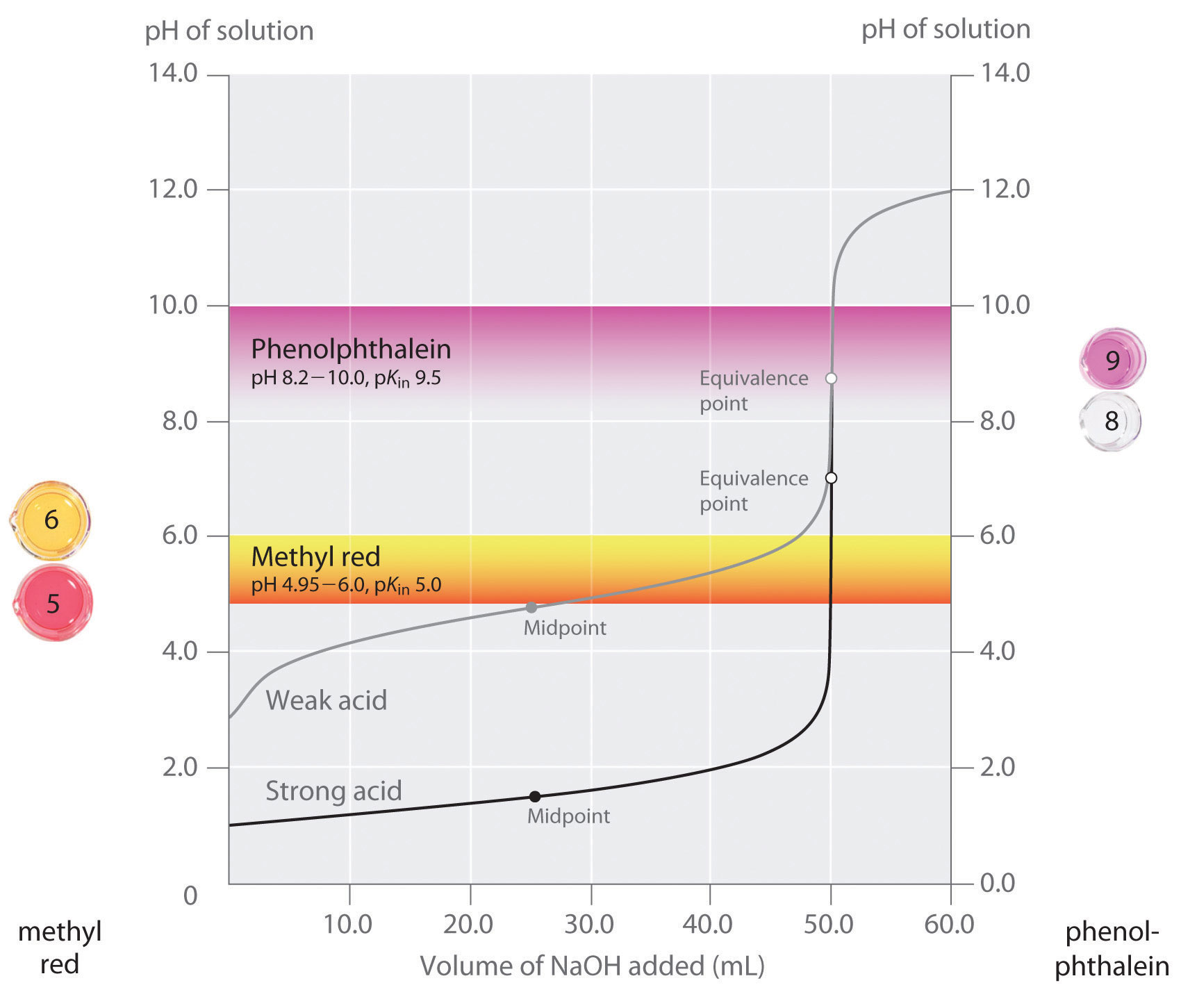
Titration Value . Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. What is the equivalence point of a titration. What are titrant and analyte. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. List the known values and plan the problem. Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Learn the titration graph and titration equation.
from saylordotorg.github.io
The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. What are titrant and analyte. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Learn the titration graph and titration equation. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. List the known values and plan the problem. Our titration calculator will help you never have to ask how do i calculate titrations? again. What is the equivalence point of a titration.
AcidBase Titrations
Titration Value A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Our titration calculator will help you never have to ask how do i calculate titrations? again. Learn the titration graph and titration equation. List the known values and plan the problem. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. What are titrant and analyte. What is the equivalence point of a titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Value As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Our titration calculator will help you never have to ask how do i calculate. Titration Value.
From www.slideserve.com
PPT Titration Curves PowerPoint Presentation, free download ID4273257 Titration Value A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. What is the equivalence point. Titration Value.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Titration Value List the known values and plan the problem. What is the equivalence point of a titration. Learn the titration graph and titration equation. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. What are titrant and analyte. Our titration calculator will help you never have to ask how do. Titration Value.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Value A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Learn the titration graph and titration equation. What are titrant and analyte. What is the equivalence point of a titration. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of. Titration Value.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Value A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Titration is the slow addition of one solution. Titration Value.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Value List the known values and plan the problem. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. What are titrant and analyte. Learn the titration graph. Titration Value.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Value List the known values and plan the problem. What are titrant and analyte. Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a volumetric technique in which a solution of. Titration Value.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download Titration Value A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added. Titration Value.
From joimexzcj.blob.core.windows.net
Ph Titration Definition at Michele Esposito blog Titration Value Learn the titration graph and titration equation. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. What are titrant and analyte. The basic process involves. Titration Value.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration Value What are titrant and analyte. Learn the titration graph and titration equation. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. List the known values and plan the problem. The basic process involves adding a standard solution of one reagent to a known amount of. Titration Value.
From www.youtube.com
Year 12 Chemistry Revision Titrating Using the Burette to make an Titration Value What is the equivalence point of a titration. Our titration calculator will help you never have to ask how do i calculate titrations? again. List the known values and plan the problem. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is the slow addition. Titration Value.
From exyouyqrf.blob.core.windows.net
Titration Apparatus And Uses at Nicole Fain blog Titration Value List the known values and plan the problem. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. What is the equivalence point of a titration. Learn the titration graph and titration equation. A titration is a volumetric technique in which a solution of one reactant (the. Titration Value.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Titration Value The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Our titration calculator will help you never have to ask how do i calculate titrations? again. Learn the titration graph and titration equation. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. Titration Value.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titration Value List the known values and plan the problem. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. What are titrant and analyte. Our titration calculator will help you never have to ask how do i calculate titrations? again. As seen in the chapter on the. Titration Value.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Value As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Learn the titration graph and titration equation. The basic process involves adding a standard solution of one. Titration Value.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation Titration Value List the known values and plan the problem. Learn the titration graph and titration equation. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. What are. Titration Value.
From www.easybiologyclass.com
What is Titration Curve? What is pKa? EasyBiologyClass Titration Value What is the equivalence point of a titration. What are titrant and analyte. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent.. Titration Value.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Value A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Learn the titration graph and. Titration Value.
From www.researchgate.net
Titre values obtained from the visual titration for samples A to H Titration Value Learn the titration graph and titration equation. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. List the known values and plan the problem. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. As. Titration Value.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Value What is the equivalence point of a titration. What are titrant and analyte. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Learn the titration graph and titration equation. Titration is the slow addition of one solution of a known concentration (called a titrant) to a. Titration Value.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Value Our titration calculator will help you never have to ask how do i calculate titrations? again. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. What is the equivalence point of a titration. A titration is a volumetric technique in which a solution of one reactant. Titration Value.
From www.youtube.com
Titration Calculations YouTube Titration Value Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. List the known values and plan the problem. As seen in the. Titration Value.
From www.writework.com
Titration of amino acids WriteWork Titration Value List the known values and plan the problem. What are titrant and analyte. Our titration calculator will help you never have to ask how do i calculate titrations? again. Learn the titration graph and titration equation. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow. Titration Value.
From joieldmit.blob.core.windows.net
How Is Titration Used In Medicine at Shelia Wiley blog Titration Value Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. List the known values. Titration Value.
From www.chegg.com
Solved The graph shows the titration curves of a strong acid Titration Value A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Our titration calculator will help you never have. Titration Value.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Titration Value Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Learn the titration graph and titration equation. The basic process involves adding a standard solution of one reagent to a known amount of the. Titration Value.
From www.youtube.com
Ch.3 Amino Acid 6 Titration curve of Acidic A.A (Aspartic Acid)and net Titration Value Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Learn the titration graph and titration equation. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. List the known values and plan. Titration Value.
From general.chemistrysteps.com
General Chemistry Archives Page 8 of 18 Chemistry Steps Titration Value Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. List the known values and plan the problem. Our titration calculator will help you never have to ask how do i calculate titrations? again. What is the equivalence point of a titration. A titration is a. Titration Value.
From webmis.highland.cc.il.us
AcidBase Titrations Titration Value Our titration calculator will help you never have to ask how do i calculate titrations? again. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. Titration Value.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Value Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. List the known values and plan the problem. Our titration calculator will help you never have. Titration Value.
From chemistryguru.com.sg
Titration Curve of Amino Acid Titration Value As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Learn the titration graph and titration equation. What is the equivalence point of a titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. Titration Value.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Value Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Learn the titration graph and titration equation. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. What are titrant and analyte. As seen in the. Titration Value.
From saylordotorg.github.io
AcidBase Titrations Titration Value Learn the titration graph and titration equation. Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. What are titrant and analyte.. Titration Value.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Value Our titration calculator will help you never have to ask how do i calculate titrations? again. What are titrant and analyte. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. What is the equivalence point of a titration. A titration is a volumetric technique in which a solution of. Titration Value.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Value Learn the titration graph and titration equation. List the known values and plan the problem. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. What is the equivalence point of a titration. As seen in the chapter. Titration Value.