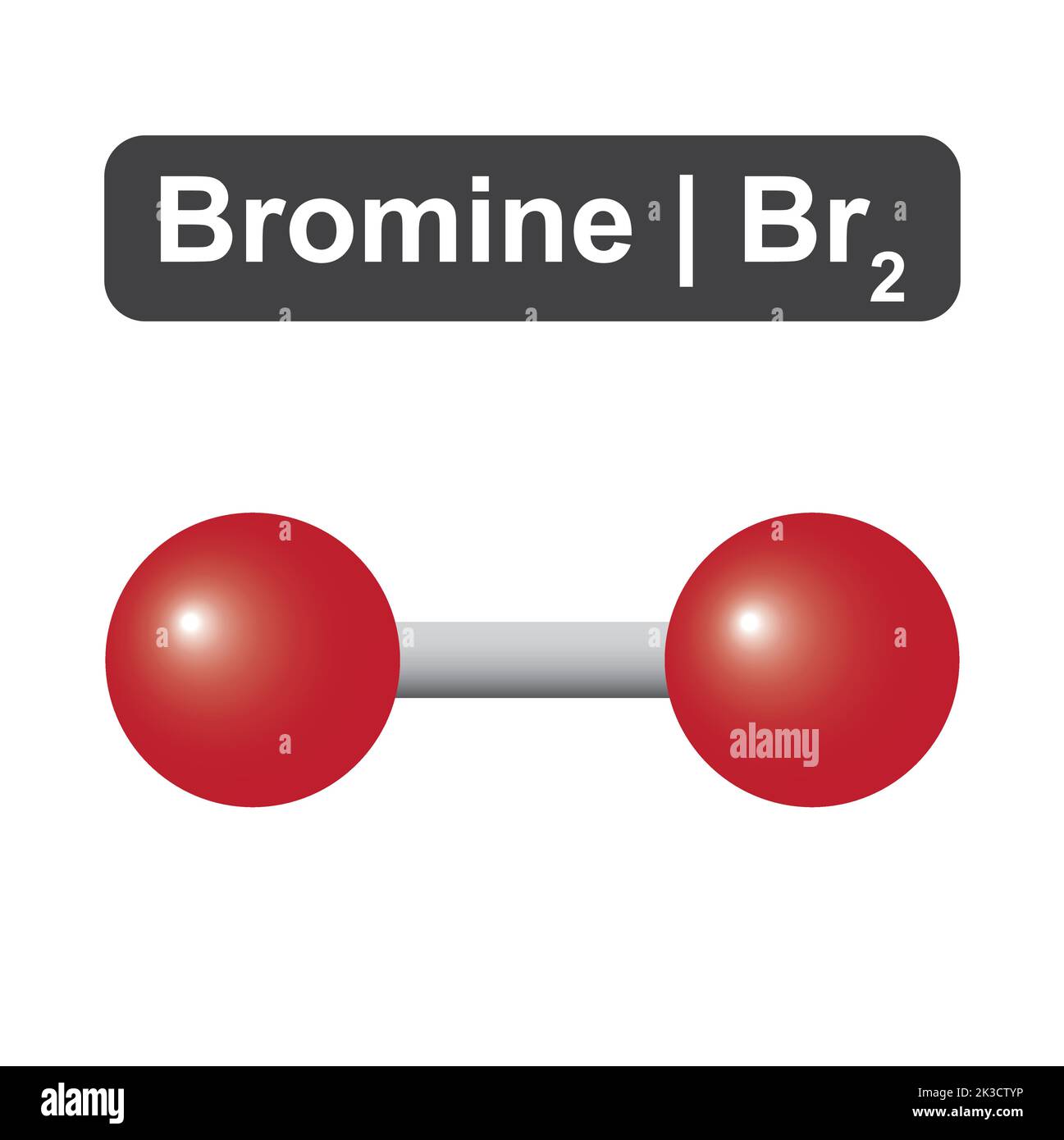
Bromine Gas Imfa . The phase in which a. They are all symetric homonuclear. Because the atoms on either side of the covalent bond are. Explain the reason why iodine is a solid, bromine is a liquid, and fluorine is a gas at room temperature. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature and pressure. Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena, as. The phase in which a. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Elemental bromine has two bromine atoms covalently bonded to each other.
from www.alamy.com
Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena, as. Because the atoms on either side of the covalent bond are. The phase in which a. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Explain the reason why iodine is a solid, bromine is a liquid, and fluorine is a gas at room temperature. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature and pressure. They are all symetric homonuclear. The phase in which a. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Elemental bromine has two bromine atoms covalently bonded to each other.
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock
Bromine Gas Imfa They are all symetric homonuclear. They are all symetric homonuclear. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature and pressure. The phase in which a. Explain the reason why iodine is a solid, bromine is a liquid, and fluorine is a gas at room temperature. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Because the atoms on either side of the covalent bond are. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. The phase in which a. Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena, as. Elemental bromine has two bromine atoms covalently bonded to each other.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Gas Imfa The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Elemental bromine. Bromine Gas Imfa.
From www.alamy.com
Bromine liquid and gas hires stock photography and images Alamy Bromine Gas Imfa The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena, as. The phase in which a. Explain the reason. Bromine Gas Imfa.
From gradegorilla.com
Gradegorilla Chemistry Bromine Gas Imfa These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature and pressure. Because the atoms on either side of the covalent bond are. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up. Bromine Gas Imfa.
From fphoto.photoshelter.com
science element bromine Fundamental Photographs The Art of Science Bromine Gas Imfa The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. The phase in which a. They are all symetric homonuclear. The phase in which a. The differences in the properties of a solid, liquid, or gas reflect the strengths of the. Bromine Gas Imfa.
From www.sciencephoto.com
Bromine gas Stock Image A150/0399 Science Photo Library Bromine Gas Imfa The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. The phase in which a. Explain the reason why iodine is a solid, bromine is a liquid, and fluorine is a gas at room temperature. The differences in the properties of. Bromine Gas Imfa.
From sciencephotogallery.com
Bromine Gas Diffusion 2 by Martyn F. Chillmaid/science Photo Library Bromine Gas Imfa The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. The phase in which a. The phase in which a. Explain the reason why iodine is a solid, bromine is a liquid, and fluorine is a gas at room temperature. Because. Bromine Gas Imfa.
From fphoto.photoshelter.com
science exothermic reaction sodium bromine Fundamental Photographs Bromine Gas Imfa The phase in which a. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature and pressure. Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena, as. The phase in which a. Elemental bromine has two bromine. Bromine Gas Imfa.
From slideplayer.com
Phase Changes and Intermolecular Forces ppt download Bromine Gas Imfa The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Elemental bromine has two bromine atoms covalently bonded to each other. Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to. Bromine Gas Imfa.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Bromine Gas Imfa They are all symetric homonuclear. Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena, as. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. The differences in. Bromine Gas Imfa.
From sciencephotogallery.com
Bromine Gas by Science Photo Library Bromine Gas Imfa Explain the reason why iodine is a solid, bromine is a liquid, and fluorine is a gas at room temperature. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature and pressure. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces. Bromine Gas Imfa.
From www.numerade.com
SOLVED The reaction of methane with bromine gas is CH4(g) + Br2(g) â Bromine Gas Imfa The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Explain the reason why iodine is a solid, bromine is a liquid, and fluorine is a gas at room temperature. The phase in which a. The phase in which a. Because. Bromine Gas Imfa.
From www.forensicsdetectors.com
Best Bromine Gas Detector (2024 update) Forensics Detectors Bromine Gas Imfa These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature and pressure. They are all symetric homonuclear. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Explain the reason why. Bromine Gas Imfa.
From www.alamy.com
Bromine diffusion hires stock photography and images Alamy Bromine Gas Imfa The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Elemental bromine. Bromine Gas Imfa.
From www.semanticscholar.org
Figure 1 from Behavioral and Neuronal Effects of Inhaled Bromine Gas Bromine Gas Imfa Because the atoms on either side of the covalent bond are. Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena, as. The phase in which a. They are all symetric homonuclear. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under. Bromine Gas Imfa.
From www.sciencephoto.com
Bromine gas Stock Image C055/5552 Science Photo Library Bromine Gas Imfa The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Explain the reason why iodine is a solid, bromine is a liquid, and fluorine is a gas at room temperature. The phase in which a. Because the atoms on either side. Bromine Gas Imfa.
From edu.rsc.org
Diffusion of gases a safer alternative to bromine Experiment RSC Bromine Gas Imfa The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena, as. Elemental bromine has two bromine atoms covalently bonded. Bromine Gas Imfa.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Bromine Gas Imfa Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena, as. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature and pressure. The phase in which a. Elemental bromine has two bromine atoms covalently bonded to each. Bromine Gas Imfa.
From pubs.acs.org
Detection of Bromine by ICPoaToFMS Following Photochemical Vapor Bromine Gas Imfa Explain the reason why iodine is a solid, bromine is a liquid, and fluorine is a gas at room temperature. Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena, as. They are all symetric homonuclear. Elemental bromine has two bromine atoms covalently bonded to each other. Because. Bromine Gas Imfa.
From www.sciencephoto.com
Diffusion of Bromine gas Stock Image A500/0748 Science Photo Library Bromine Gas Imfa Because the atoms on either side of the covalent bond are. Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena, as. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make. Bromine Gas Imfa.
From www.youtube.com
Write the Formula for Bromine gas YouTube Bromine Gas Imfa The phase in which a. Explain the reason why iodine is a solid, bromine is a liquid, and fluorine is a gas at room temperature. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. The differences in the properties of. Bromine Gas Imfa.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Bromine Gas Imfa Elemental bromine has two bromine atoms covalently bonded to each other. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. The phase in which a. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine. Bromine Gas Imfa.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Bromine Gas Imfa Because the atoms on either side of the covalent bond are. They are all symetric homonuclear. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. The phase in which a. Elemental bromine has two bromine atoms covalently bonded to each. Bromine Gas Imfa.
From www.alamy.com
Bromine reaction. Bromine gas (orange) forming as the product of a Bromine Gas Imfa Because the atoms on either side of the covalent bond are. Explain the reason why iodine is a solid, bromine is a liquid, and fluorine is a gas at room temperature. The phase in which a. They are all symetric homonuclear. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between. Bromine Gas Imfa.
From slideplayer.com
Phase Changes and Intermolecular Forces ppt download Bromine Gas Imfa Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena, as. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature and pressure. The phase in which a. Because the atoms on either side of the covalent bond. Bromine Gas Imfa.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Bromine Gas Imfa The phase in which a. The phase in which a. They are all symetric homonuclear. The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Imfs are the various forces of attraction that may exist between the atoms and molecules of. Bromine Gas Imfa.
From fineartamerica.com
Bromine Gas Diffusion Photograph by Martyn F. Chillmaid/science Photo Bromine Gas Imfa The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Elemental bromine has two bromine atoms covalently bonded to each other. The phase in which a. Imfs are the various forces of attraction that may exist between the atoms and molecules. Bromine Gas Imfa.
From fphoto.photoshelter.com
science element bromine Fundamental Photographs The Art of Science Bromine Gas Imfa Explain the reason why iodine is a solid, bromine is a liquid, and fluorine is a gas at room temperature. The phase in which a. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature and pressure. The differences in the properties of a solid, liquid, or gas reflect the. Bromine Gas Imfa.
From www.slideserve.com
PPT Matter and Change PowerPoint Presentation, free download ID9660645 Bromine Gas Imfa They are all symetric homonuclear. Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena, as. Elemental bromine has two bromine atoms covalently bonded to each other. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature and. Bromine Gas Imfa.
From fineartamerica.com
Bromine Gas Diffusion Photograph by Martyn F. Chillmaid/science Photo Bromine Gas Imfa The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature and pressure. Because the atoms on either side of the covalent. Bromine Gas Imfa.
From fphoto.photoshelter.com
science exothermic reaction sodium bromine Fundamental Photographs Bromine Gas Imfa Because the atoms on either side of the covalent bond are. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature and pressure. The phase in which a. Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena,. Bromine Gas Imfa.
From www.mdpi.com
IJMS Free FullText Behavioral and Neuronal Effects of Inhaled Bromine Gas Imfa They are all symetric homonuclear. The phase in which a. Explain the reason why iodine is a solid, bromine is a liquid, and fluorine is a gas at room temperature. Imfs are the various forces of attraction that may exist between the atoms and molecules of a substance due to electrostatic phenomena, as. Elemental bromine has two bromine atoms covalently. Bromine Gas Imfa.
From www.savemyexams.com
Simple Diffusion Experiments Oxford AQA IGCSE Chemistry Revision Bromine Gas Imfa The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. They are all symetric homonuclear. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature and pressure. The differences in the. Bromine Gas Imfa.
From periodictable.com
Gas in a bulb, a sample of the element Bromine in the Periodic Table Bromine Gas Imfa The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Because the atoms on either side of the covalent bond are. The phase in which a. Imfs are the various forces of attraction that may exist between the atoms and molecules. Bromine Gas Imfa.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Bromine Gas Imfa The differences in the properties of a solid, liquid, or gas reflect the strengths of the attractive forces between the atoms, molecules, or ions that make up each phase. Elemental bromine has two bromine atoms covalently bonded to each other. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature. Bromine Gas Imfa.
From fphoto.photoshelter.com
bromine phases transition chemistry element Fundamental Photographs Bromine Gas Imfa Elemental bromine has two bromine atoms covalently bonded to each other. Because the atoms on either side of the covalent bond are. These intermolecular interactions are strong enough to favor the condensed states for bromine and iodine under normal conditions of temperature and pressure. The differences in the properties of a solid, liquid, or gas reflect the strengths of the. Bromine Gas Imfa.