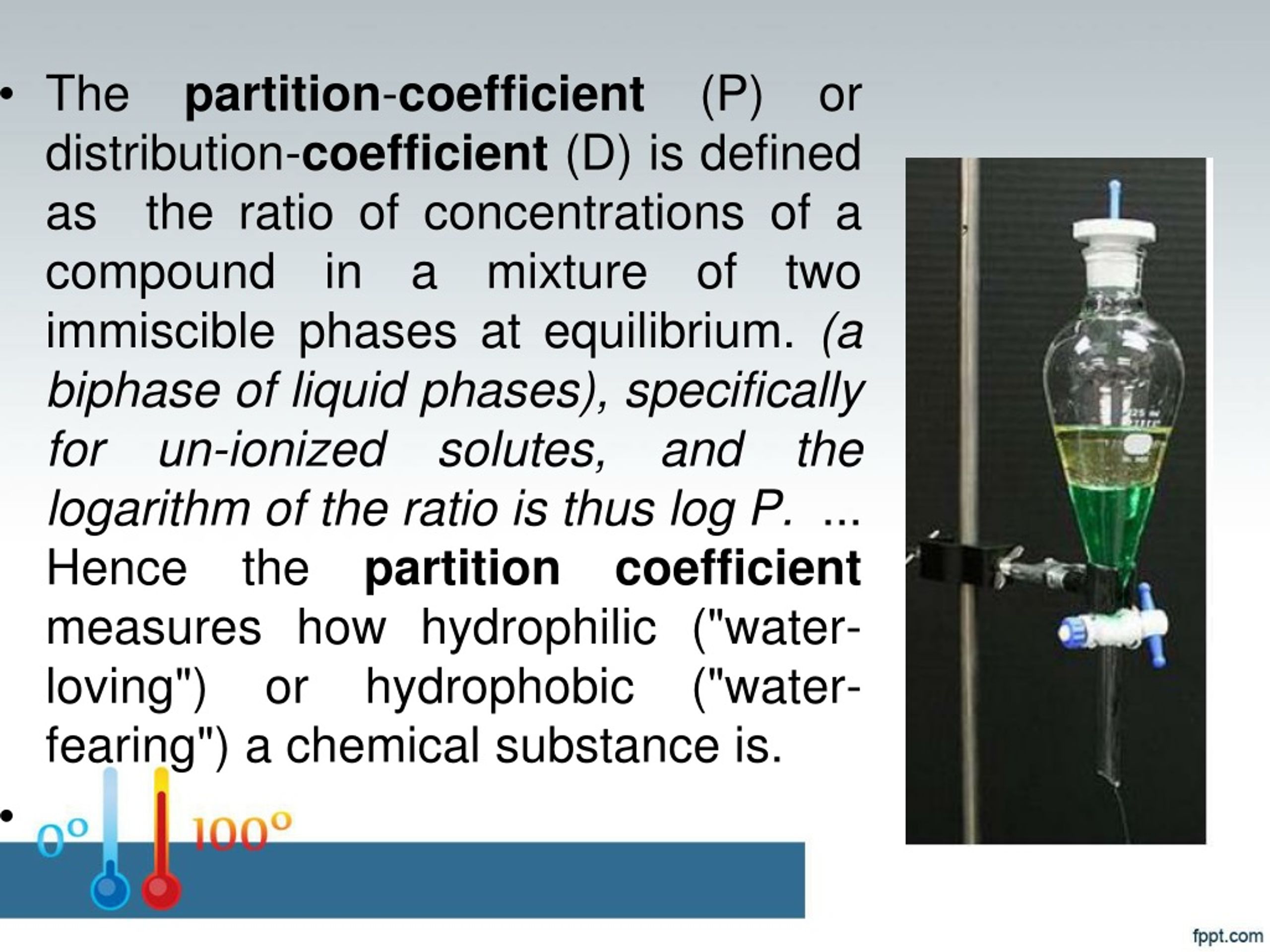
Define Partition Coefficient . The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other. A partition coefficient is the ratio of a solute in two different solvents. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. The partition coefficient is defined as the ratio of the concentration of a solute in the organic phase to its concentration in the water phase. Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. A partition coefficient is the ratio of the concentration of a substance in one medium or phase (c 1) to the concentration in a second. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate.
from www.slideserve.com
Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. A partition coefficient is the ratio of the concentration of a substance in one medium or phase (c 1) to the concentration in a second. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. The partition coefficient is defined as the ratio of the concentration of a solute in the organic phase to its concentration in the water phase. A partition coefficient is the ratio of a solute in two different solvents.
PPT lab no.(5) Partition coefficients PowerPoint Presentation, free
Define Partition Coefficient The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. The partition coefficient is defined as the ratio of the concentration of a solute in the organic phase to its concentration in the water phase. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. A partition coefficient is the ratio of a solute in two different solvents. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. A partition coefficient is the ratio of the concentration of a substance in one medium or phase (c 1) to the concentration in a second.
From www.researchgate.net
Composition, partition coefficient, slope of liquidus and solutal Define Partition Coefficient A partition coefficient is the ratio of the concentration of a substance in one medium or phase (c 1) to the concentration in a second. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other. A partition coefficient is the ratio of a solute in two. Define Partition Coefficient.
From www.chegg.com
Solved Sample Questions 1. Define the partition coefficient Define Partition Coefficient A partition coefficient is the ratio of a solute in two different solvents. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. The partition coefficient is defined as the ratio of the concentration of a solute in the organic phase to its concentration in the water phase. A partition coefficient is the ratio. Define Partition Coefficient.
From www.youtube.com
Partition coefficients YouTube Define Partition Coefficient A partition coefficient is the ratio of the concentration of a substance in one medium or phase (c 1) to the concentration in a second. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. The ratio of a. Define Partition Coefficient.
From www.youtube.com
Partition coefficient Top 11 Facts YouTube Define Partition Coefficient Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. A partition coefficient is the ratio of the concentration of a substance in one medium or phase (c 1) to the concentration in a second. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents. Define Partition Coefficient.
From www.slideserve.com
PPT GOLDSCHMIDT’S RULES PowerPoint Presentation ID310825 Define Partition Coefficient The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. The partition coefficient is defined as the ratio of the concentration of a solute in the organic phase to its concentration in the water phase. The ratio of a. Define Partition Coefficient.
From www.chegg.com
Solved 1. Explain partitioning coefficient and define the Define Partition Coefficient Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. A partition coefficient is the ratio of a solute in two different solvents. The partition coefficient is defined as the ratio of the concentration of a solute in the organic phase to its concentration in the water phase. The partition coefficient (k pc) is. Define Partition Coefficient.
From relationshipbetween.com
Difference Between Partition Coefficient And Distribution Coefficient Define Partition Coefficient The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. A partition coefficient is the ratio of a solute in two different solvents. The. Define Partition Coefficient.
From pubs.acs.org
OctanolWater Partition Coefficient Measurement by a Simple 1H NMR Define Partition Coefficient Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. A partition coefficient is the ratio of a solute in two different solvents. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. The. Define Partition Coefficient.
From mungfali.com
Partition Coefficient Phase Diagram Define Partition Coefficient The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often. Define Partition Coefficient.
From www.slideserve.com
PPT Partition Coefficients PowerPoint Presentation, free download Define Partition Coefficient The partition coefficient is defined as the ratio of the concentration of a solute in the organic phase to its concentration in the water phase. Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents. Define Partition Coefficient.
From www.slideserve.com
PPT Partition Coefficients PowerPoint Presentation, free download Define Partition Coefficient The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other. A partition coefficient is the ratio of the concentration of a substance in one medium or phase (c 1) to the concentration in a second. A partition coefficient is the ratio of a solute in two. Define Partition Coefficient.
From mungfali.com
Partition Coefficient Phase Diagram Define Partition Coefficient The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other. The partition coefficient (k pc) is. Define Partition Coefficient.
From slidetodoc.com
Partition Coefficients Lecture 26 The Partition Coefficient Geochemists Define Partition Coefficient The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. A partition coefficient is the ratio of a solute in two different solvents.. Define Partition Coefficient.
From www.chegg.com
Solved In Chromatography, The Partition Coefficient, K, D... Define Partition Coefficient The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other. Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. A partition coefficient is. Define Partition Coefficient.
From www.hhi.fraunhofer.de
Transform Coefficient Partitioning Define Partition Coefficient The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. The partition coefficient is defined as the ratio of the concentration of a solute in the organic phase to its concentration in the water phase. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. The partition coefficient (k pc). Define Partition Coefficient.
From www.slideshare.net
Partition coefficient Define Partition Coefficient Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. A partition coefficient is the ratio of a solute in two different solvents. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different. Define Partition Coefficient.
From www.slideserve.com
PPT Lipinski’s rule of five PowerPoint Presentation, free download Define Partition Coefficient The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other. The partition coefficient is defined. Define Partition Coefficient.
From www.researchgate.net
Partition coefficients. Download Table Define Partition Coefficient The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. A partition coefficient is the ratio of the concentration of a substance in one medium or phase (c 1) to the concentration in a second. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact. Define Partition Coefficient.
From www.researchgate.net
Partitioning coefficients, with values calculated for each sample and Define Partition Coefficient Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. The partition coefficient is defined as the ratio of the concentration of a solute in the organic phase to its concentration in the water phase. A partition coefficient is the ratio of the concentration of a substance in one medium or phase (c 1). Define Partition Coefficient.
From www.scribd.com
Partition Coefficient Edited Solvent Solubility Define Partition Coefficient Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. A partition coefficient is the ratio of a solute in two different solvents. The. Define Partition Coefficient.
From www.slideserve.com
PPT Lipinski’s rule of five PowerPoint Presentation ID2937410 Define Partition Coefficient A partition coefficient is the ratio of the concentration of a substance in one medium or phase (c 1) to the concentration in a second. The partition coefficient is defined as the ratio of the concentration of a solute in the organic phase to its concentration in the water phase. The partition coefficient (k pc) is the ratio of the. Define Partition Coefficient.
From www.researchgate.net
Graphical presentation of partition coefficients... Download Define Partition Coefficient Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. The partition coefficient is defined as the ratio of the concentration of a solute in the organic phase to its concentration in the water phase. Partition coefficients are described as. Define Partition Coefficient.
From www.slideserve.com
PPT lab no.(5) Partition coefficients PowerPoint Presentation, free Define Partition Coefficient A partition coefficient is the ratio of the concentration of a substance in one medium or phase (c 1) to the concentration in a second. The partition coefficient is defined as the ratio of the concentration of a solute in the organic phase to its concentration in the water phase. A partition coefficient is the ratio of a solute in. Define Partition Coefficient.
From www.researchgate.net
Plot of experimentally determined Partition coefficient.( logP Expt Define Partition Coefficient The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. The partition coefficient is defined as the ratio of the concentration of a. Define Partition Coefficient.
From pubs.acs.org
Calculating Partition Coefficients of Small Molecules in Octanol/Water Define Partition Coefficient The partition coefficient is defined as the ratio of the concentration of a solute in the organic phase to its concentration in the water phase. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other. A partition coefficient is the ratio of the concentration of a. Define Partition Coefficient.
From www.researchgate.net
Partition coefficient of CO 3 2 groups between coexisting fluid and Define Partition Coefficient Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. The ratio of a compound’s unionized species concentrations in a mixture of two. Define Partition Coefficient.
From www.researchgate.net
Plot of the partition coefficient as a function of the temperature of Define Partition Coefficient A partition coefficient is the ratio of the concentration of a substance in one medium or phase (c 1) to the concentration in a second. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. A partition coefficient is the ratio of a solute in two different solvents. The partition coefficient (k pc) is. Define Partition Coefficient.
From www.researchgate.net
Calculations of partition coefficient and diffusive velocity. a, The Define Partition Coefficient Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. A partition coefficient is the ratio of a solute in two different solvents. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. The. Define Partition Coefficient.
From www.numerade.com
SOLVED What is the Distribution Coefficient (Partition Coefficient Define Partition Coefficient The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. A partition coefficient is the ratio of the concentration of a substance in one medium or phase (c 1) to the concentration in a second. Partition coefficients are described as. Define Partition Coefficient.
From www.youtube.com
Partition coefficient and element compatibility YouTube Define Partition Coefficient The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. The partition coefficient is defined as the ratio of the concentration of a solute in the organic phase to its concentration in the water phase. A partition coefficient is the ratio of a solute in two different solvents. Solubility (grams per 100 ml) or molarity concentrations. Define Partition Coefficient.
From www.chegg.com
4. (a) Define the term partition coefficient. (b) Define Partition Coefficient The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. A partition coefficient is the ratio of the concentration of a substance in. Define Partition Coefficient.
From rotachrom.com
Understanding the Partition Coefficient Rotachrom Technologies Define Partition Coefficient Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. A partition coefficient is the ratio of a solute in two different solvents. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other. The partition coefficient (k pc) is the. Define Partition Coefficient.
From www.youtube.com
Partition Coefficient Organic Chemistry Lab lecture YouTube Define Partition Coefficient A partition coefficient is the ratio of a solute in two different solvents. A partition coefficient is the ratio of the concentration of a substance in one medium or phase (c 1) to the concentration in a second. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. The partition coefficient is defined as the ratio. Define Partition Coefficient.
From www.slideserve.com
PPT Partition Coefficients PowerPoint Presentation, free download Define Partition Coefficient Partition coefficients are described as the concentration ratio of a chemical amidst the two media at. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. The partition coefficient (k pc) is. Define Partition Coefficient.
From www.slideserve.com
PPT Separation Techniques PowerPoint Presentation, free download ID Define Partition Coefficient The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. A partition coefficient is the ratio of a solute in two different solvents. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different. Define Partition Coefficient.