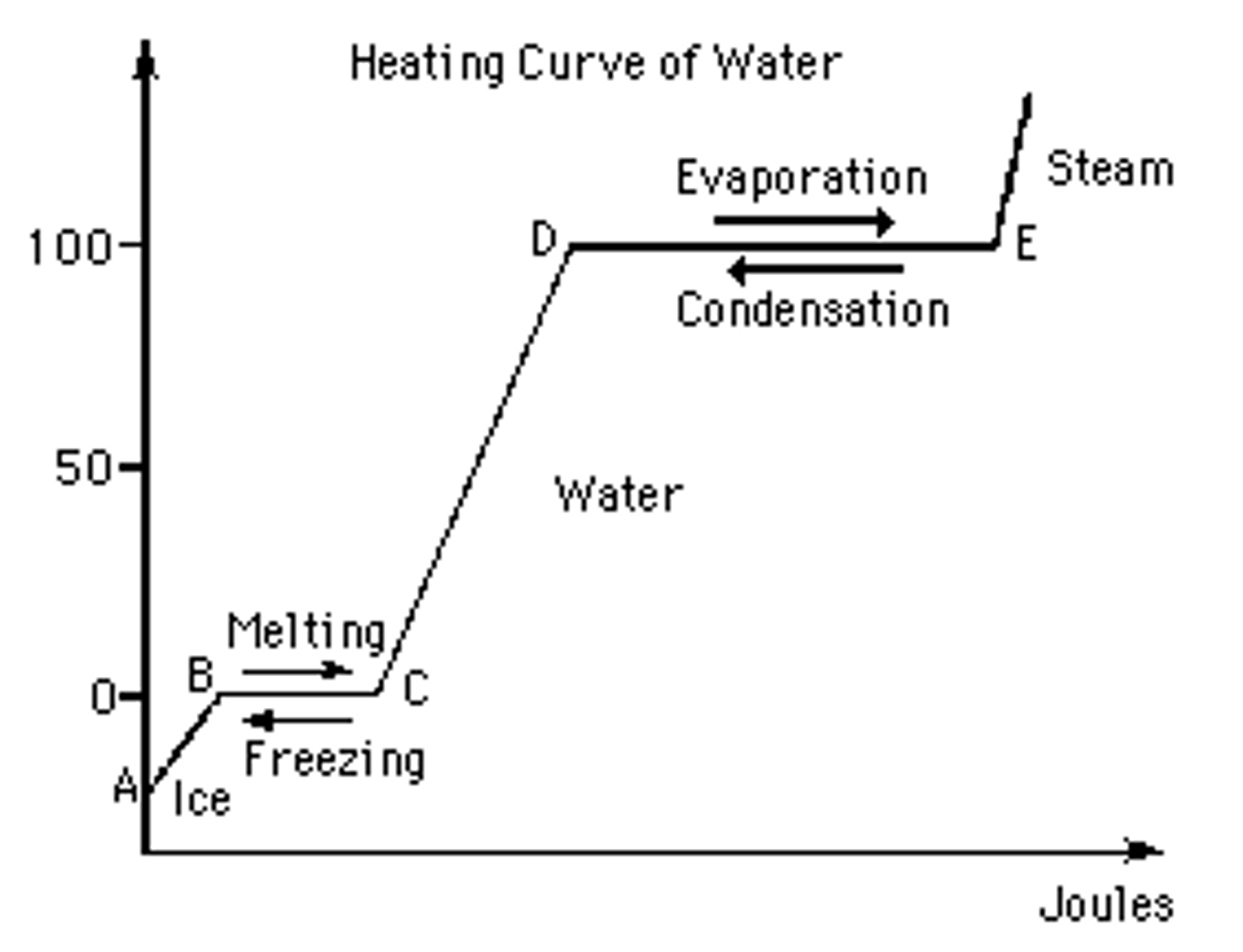
Heating Cooling Curve Of Water . As heat is steadily added to the. These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: Different substances have different melting points and boiling points, but the shapes of their heating. The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. Heating and cooling curves (the basics) what happens to the temperature of a block of ice when you put a bunsen burner underneath it? The ice is in a closed container. A cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected temperature. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. For water, this temperature is 100°c because the boiling point for water is 100°c. A heating curve for water.
from classdbjacquelyn.z21.web.core.windows.net
Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The ice is in a closed container. The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: A cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected temperature. Heating and cooling curves (the basics) what happens to the temperature of a block of ice when you put a bunsen burner underneath it? This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as. A heating curve for water. As heat is steadily added to the. For water, this temperature is 100°c because the boiling point for water is 100°c.
Heat Curve Of Water
Heating Cooling Curve Of Water These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: A cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected temperature. Heating and cooling curves (the basics) what happens to the temperature of a block of ice when you put a bunsen burner underneath it? A heating curve for water. For water, this temperature is 100°c because the boiling point for water is 100°c. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. As heat is steadily added to the. Different substances have different melting points and boiling points, but the shapes of their heating. The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as. The ice is in a closed container.
From slidetodoc.com
CALCULATING ENERGY CHANGES HEATING CURVE OF WATER COOLING Heating Cooling Curve Of Water Different substances have different melting points and boiling points, but the shapes of their heating. The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. As heat is steadily added to the. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well. Heating Cooling Curve Of Water.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating Cooling Curve Of Water These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: Different substances have different melting points and boiling points, but the shapes of their heating. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as. Imagine that you have a block of. Heating Cooling Curve Of Water.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/ Heating Cooling Curve Of Water The ice is in a closed container. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. For water, this temperature is 100°c because the boiling point for water is 100°c. Different substances have different melting points and boiling points, but the shapes of their heating.. Heating Cooling Curve Of Water.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download Heating Cooling Curve Of Water Different substances have different melting points and boiling points, but the shapes of their heating. As heat is steadily added to the. A cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected temperature. These curves show a visualization of how phase changes occur (via adding or removing heat) heating. Heating Cooling Curve Of Water.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation Heating Cooling Curve Of Water Different substances have different melting points and boiling points, but the shapes of their heating. As heat is steadily added to the. Heating and cooling curves (the basics) what happens to the temperature of a block of ice when you put a bunsen burner underneath it? A heating curve for water. This plot of temperature shows what happens to a. Heating Cooling Curve Of Water.
From www.youtube.com
Heating Curve of Water YouTube Heating Cooling Curve Of Water Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. A cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze. Heating Cooling Curve Of Water.
From quizlet.com
Heating Curve for Water Diagram Quizlet Heating Cooling Curve Of Water The ice is in a closed container. A heating curve for water. For water, this temperature is 100°c because the boiling point for water is 100°c. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as. The energy changes that occur during phase changes can be quantified by using. Heating Cooling Curve Of Water.
From chem.libretexts.org
12.7 Heating Curve for Water Chemistry LibreTexts Heating Cooling Curve Of Water These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. A cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at. Heating Cooling Curve Of Water.
From quizzschoolhernandez.z19.web.core.windows.net
Heating Curve Of Water Explained Heating Cooling Curve Of Water Heating and cooling curves (the basics) what happens to the temperature of a block of ice when you put a bunsen burner underneath it? These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and. Heating Cooling Curve Of Water.
From www.youtube.com
Heating and Cooling Curve for Water YouTube Heating Cooling Curve Of Water The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. As heat is steadily added to the. The ice is in a closed container. A heating curve for water. Different substances have different melting points and boiling points, but the shapes of their heating. For water, this temperature is 100°c because the. Heating Cooling Curve Of Water.
From slidetodoc.com
CALCULATING ENERGY CHANGES HEATING CURVE OF WATER COOLING Heating Cooling Curve Of Water The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. As heat is steadily added to the. The ice is in a closed container. These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: Heating and cooling curves (the basics) what happens to the temperature. Heating Cooling Curve Of Water.
From www.slideserve.com
PPT Heating Curves and Thermodynamics PowerPoint Presentation, free Heating Cooling Curve Of Water Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: A heating curve for water. Different substances have different melting points and boiling points, but the shapes of their. Heating Cooling Curve Of Water.
From quizzzonevalencia.z21.web.core.windows.net
Heating Curve Of Water Explained Heating Cooling Curve Of Water Heating and cooling curves (the basics) what happens to the temperature of a block of ice when you put a bunsen burner underneath it? This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as. The ice is in a closed container. Different substances have different melting points and boiling. Heating Cooling Curve Of Water.
From printablelibagnames.z13.web.core.windows.net
Heating Curve Of Water Explained Heating Cooling Curve Of Water Different substances have different melting points and boiling points, but the shapes of their heating. The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. A cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected temperature. The ice is in a. Heating Cooling Curve Of Water.
From classdbjacquelyn.z21.web.core.windows.net
Heat Curve Of Water Heating Cooling Curve Of Water The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: A cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected temperature. For water, this. Heating Cooling Curve Of Water.
From www.youtube.com
Heating Curve of Water YouTube Heating Cooling Curve Of Water The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. Heating and cooling curves (the basics) what happens to the temperature of a block of ice when you put a bunsen burner underneath it? This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm. Heating Cooling Curve Of Water.
From ar.inspiredpencil.com
Graph Heating Curve For Water Heating Cooling Curve Of Water For water, this temperature is 100°c because the boiling point for water is 100°c. A heating curve for water. Different substances have different melting points and boiling points, but the shapes of their heating. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. These curves. Heating Cooling Curve Of Water.
From www.youtube.com
Heating and Cooling Curve of Water YouTube Heating Cooling Curve Of Water For water, this temperature is 100°c because the boiling point for water is 100°c. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: The energy changes that occur. Heating Cooling Curve Of Water.
From www.slideserve.com
PPT Chapter 1 PowerPoint Presentation, free download ID5006719 Heating Cooling Curve Of Water Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Heating and cooling curves (the basics) what happens to the temperature of a block of ice when you put a bunsen burner underneath it? These curves show a visualization of how phase changes occur (via adding. Heating Cooling Curve Of Water.
From watercoolingsengihi.blogspot.com
Water Cooling Water Cooling Curve Heating Cooling Curve Of Water Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Different substances have different melting points and boiling points, but the shapes of their heating. As heat is steadily added to the. The energy changes that occur during phase changes can be quantified by using a. Heating Cooling Curve Of Water.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download Heating Cooling Curve Of Water Different substances have different melting points and boiling points, but the shapes of their heating. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as. These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: As heat is steadily added to the.. Heating Cooling Curve Of Water.
From brainly.com
Draw a cooling graph of water as it passes from 20°C to 20°C. The y Heating Cooling Curve Of Water These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: A heating curve for water. The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. Heating and cooling curves (the basics) what happens to the temperature of a block of ice when you put a. Heating Cooling Curve Of Water.
From www.slideserve.com
PPT Heating and Cooling Curves of Water PowerPoint Presentation ID Heating Cooling Curve Of Water Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as. The ice is in a closed container. The energy changes that occur during phase changes. Heating Cooling Curve Of Water.
From www.youtube.com
Heating Curve and Cooling Curve of Water Enthalpy of Fusion Heating Cooling Curve Of Water Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Different substances have different melting points and boiling points, but the shapes of their heating. The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. These curves show. Heating Cooling Curve Of Water.
From www.slideserve.com
PPT Thermochemistry The heat energy of chemical reactions PowerPoint Heating Cooling Curve Of Water Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. As heat is steadily added to the. A heating curve for water. The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. These curves show a visualization of. Heating Cooling Curve Of Water.
From www.worldwisetutoring.com
Heating and Cooling Curves Heating Cooling Curve Of Water Heating and cooling curves (the basics) what happens to the temperature of a block of ice when you put a bunsen burner underneath it? Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. A heating curve for water. The ice is in a closed container.. Heating Cooling Curve Of Water.
From spmchemistry.blog.onlinetuition.com.my
Cooling Curve SPM Chemistry Heating Cooling Curve Of Water A heating curve for water. As heat is steadily added to the. The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. Different substances have different melting points and boiling points, but the shapes of their heating. Heating and cooling curves (the basics) what happens to the temperature of a block of. Heating Cooling Curve Of Water.
From www.slideserve.com
PPT Heating and Cooling Curves of Water PowerPoint Presentation, free Heating Cooling Curve Of Water These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: Heating and cooling curves (the basics) what happens to the temperature of a block of ice when you put a bunsen burner underneath it? Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c,. Heating Cooling Curve Of Water.
From scienceisntscary.wordpress.com
Image Heating Cooling Curve Of Water A cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected temperature. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The ice is in a closed container. Heating and cooling curves (the basics) what. Heating Cooling Curve Of Water.
From slidetodoc.com
Heating and cooling curve for water heated at Heating Cooling Curve Of Water As heat is steadily added to the. Heating and cooling curves (the basics) what happens to the temperature of a block of ice when you put a bunsen burner underneath it? The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. A heating curve for water. Imagine that you have a block. Heating Cooling Curve Of Water.
From ch301.cm.utexas.edu
heating curve Heating Cooling Curve Of Water A cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected temperature. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The energy changes that occur during phase changes can be quantified by using a. Heating Cooling Curve Of Water.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Heating Cooling Curve Of Water The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. As heat is steadily added to the. A heating curve for water. Heating and cooling curves (the basics) what happens to the temperature of a block of ice when you put a bunsen burner underneath it? Different substances have different melting points. Heating Cooling Curve Of Water.
From www.youtube.com
AP Video 10.6 Intro to HeatingCooling Curves & Calculations YouTube Heating Cooling Curve Of Water For water, this temperature is 100°c because the boiling point for water is 100°c. The energy changes that occur during phase changes can be quantified by using a heating or cooling curve. The ice is in a closed container. These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: Imagine that you have. Heating Cooling Curve Of Water.
From stgeorgesinstitute.org
Heating & Cooling Curves SGI MyCORE Heating Cooling Curve Of Water A heating curve for water. For water, this temperature is 100°c because the boiling point for water is 100°c. Heating and cooling curves (the basics) what happens to the temperature of a block of ice when you put a bunsen burner underneath it? Imagine that you have a block of ice that is at a temperature of −30oc − 30. Heating Cooling Curve Of Water.
From studylib.net
Heating and Cooling Curves Heating Cooling Curve Of Water For water, this temperature is 100°c because the boiling point for water is 100°c. These curves show a visualization of how phase changes occur (via adding or removing heat) heating curve: As heat is steadily added to the. The ice is in a closed container. Heating and cooling curves (the basics) what happens to the temperature of a block of. Heating Cooling Curve Of Water.