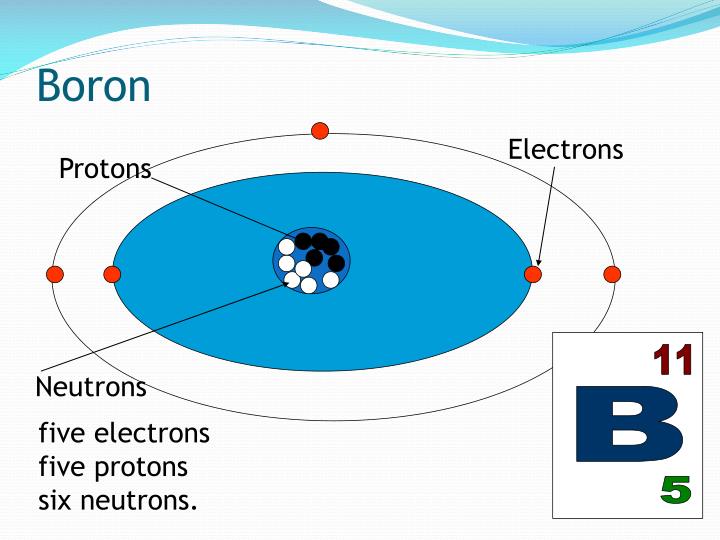
Does Boron Form A Positive Or Negative Ion . When a boron atom donates three electrons, it becomes a boron ion denoted as b 3+. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. The nature of the bond. So, the electron configuration of boron ion(b 3+) is 1s 2. Boron compounds burn with a green flame. The distinctive color leads to use in fireworks. What is the charge on its ions, and is the charge positive or negative? The energy released when an electron is added to the neutral atom and a negative ion is formed. Electronegativity (pauling scale) the tendency. The charge on the ion is. Pure boron can exist as a mixture of positive and negative boron ions. The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter.
from kladkrsvl.blob.core.windows.net
The nature of the bond. So, the electron configuration of boron ion(b 3+) is 1s 2. The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. What is the charge on its ions, and is the charge positive or negative? The distinctive color leads to use in fireworks. Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. Pure boron can exist as a mixture of positive and negative boron ions. Electronegativity (pauling scale) the tendency. The energy released when an electron is added to the neutral atom and a negative ion is formed. The charge on the ion is.
Does Boron Form A Negative Ion at Marilyn Ahner blog
Does Boron Form A Positive Or Negative Ion The charge on the ion is. The energy released when an electron is added to the neutral atom and a negative ion is formed. Boron compounds burn with a green flame. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. The distinctive color leads to use in fireworks. When a boron atom donates three electrons, it becomes a boron ion denoted as b 3+. Pure boron can exist as a mixture of positive and negative boron ions. Electronegativity (pauling scale) the tendency. What is the charge on its ions, and is the charge positive or negative? So, the electron configuration of boron ion(b 3+) is 1s 2. The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. The nature of the bond. The charge on the ion is.
From dxobgtlpw.blob.core.windows.net
Does Boron Form Ionic Bonds at Robert Spradlin blog Does Boron Form A Positive Or Negative Ion The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. The energy released when an electron is added to the neutral atom and a negative ion is formed. The nature of the bond. Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. So, the electron. Does Boron Form A Positive Or Negative Ion.
From kladkrsvl.blob.core.windows.net
Does Boron Form A Negative Ion at Marilyn Ahner blog Does Boron Form A Positive Or Negative Ion Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. The energy released when an electron is added to the neutral atom and a negative ion is formed. The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. Pure boron can exist as a mixture of. Does Boron Form A Positive Or Negative Ion.
From www.vectorstock.com
Diagram representation of the element boron Vector Image Does Boron Form A Positive Or Negative Ion The charge on the ion is. When a boron atom donates three electrons, it becomes a boron ion denoted as b 3+. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. Electronegativity (pauling scale) the tendency. The nature of the bond. Instead of forming a metallic lattice with delocalized valence electrons,. Does Boron Form A Positive Or Negative Ion.
From www.youtube.com
How to find Protons and Electrons for B 3+ (Boron Ion) YouTube Does Boron Form A Positive Or Negative Ion When a boron atom donates three electrons, it becomes a boron ion denoted as b 3+. Electronegativity (pauling scale) the tendency. The nature of the bond. The charge on the ion is. Pure boron can exist as a mixture of positive and negative boron ions. Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that. Does Boron Form A Positive Or Negative Ion.
From slideplayer.com
Atoms. ppt download Does Boron Form A Positive Or Negative Ion Boron compounds burn with a green flame. What is the charge on its ions, and is the charge positive or negative? The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. The energy released when an electron is added to the neutral atom and a negative ion is formed. When a boron atom. Does Boron Form A Positive Or Negative Ion.
From brainly.com
draw a shell model for boron identify the core and valence electrons Does Boron Form A Positive Or Negative Ion So, the electron configuration of boron ion(b 3+) is 1s 2. Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. The energy released when an electron is added to the neutral atom and a negative ion is formed. The charge on the ion is. What is the charge on its ions, and. Does Boron Form A Positive Or Negative Ion.
From loesbadmi.blob.core.windows.net
Does Boron Form Covalent Bonds at Christopher Ring blog Does Boron Form A Positive Or Negative Ion Electronegativity (pauling scale) the tendency. Pure boron can exist as a mixture of positive and negative boron ions. The nature of the bond. When a boron atom donates three electrons, it becomes a boron ion denoted as b 3+. The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. Revise the difference between. Does Boron Form A Positive Or Negative Ion.
From kladkrsvl.blob.core.windows.net
Does Boron Form A Negative Ion at Marilyn Ahner blog Does Boron Form A Positive Or Negative Ion The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. What is the charge on its ions, and is the charge positive or negative? Pure boron can exist as a mixture of positive and negative boron ions. So, the electron configuration of boron ion(b 3+) is 1s 2. Revise the difference between ionic,. Does Boron Form A Positive Or Negative Ion.
From dxobgtlpw.blob.core.windows.net
Does Boron Form Ionic Bonds at Robert Spradlin blog Does Boron Form A Positive Or Negative Ion The energy released when an electron is added to the neutral atom and a negative ion is formed. Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. When a boron atom donates three electrons,. Does Boron Form A Positive Or Negative Ion.
From kladkrsvl.blob.core.windows.net
Does Boron Form A Negative Ion at Marilyn Ahner blog Does Boron Form A Positive Or Negative Ion The charge on the ion is. The distinctive color leads to use in fireworks. The energy released when an electron is added to the neutral atom and a negative ion is formed. The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. Electronegativity (pauling scale) the tendency. Revise the difference between ionic, covalent. Does Boron Form A Positive Or Negative Ion.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Does Boron Form A Positive Or Negative Ion The energy released when an electron is added to the neutral atom and a negative ion is formed. The nature of the bond. When a boron atom donates three electrons, it becomes a boron ion denoted as b 3+. What is the charge on its ions, and is the charge positive or negative? Revise the difference between ionic, covalent and. Does Boron Form A Positive Or Negative Ion.
From loesbadmi.blob.core.windows.net
Does Boron Form Covalent Bonds at Christopher Ring blog Does Boron Form A Positive Or Negative Ion The energy released when an electron is added to the neutral atom and a negative ion is formed. The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. Electronegativity (pauling scale) the tendency. When a boron atom donates three electrons, it becomes a boron ion denoted as b 3+. Boron compounds burn with. Does Boron Form A Positive Or Negative Ion.
From www.nagwa.com
Question Video Identifying the Lewis Structure for Boron Tetrafluoride Does Boron Form A Positive Or Negative Ion The nature of the bond. So, the electron configuration of boron ion(b 3+) is 1s 2. Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. Pure boron can exist as a mixture of positive and negative boron ions. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret. Does Boron Form A Positive Or Negative Ion.
From circuitwiringchaw.z21.web.core.windows.net
Boron Lewis Diagram Does Boron Form A Positive Or Negative Ion What is the charge on its ions, and is the charge positive or negative? The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. The distinctive color leads to use in fireworks. Electronegativity (pauling scale). Does Boron Form A Positive Or Negative Ion.
From exoitjqhs.blob.core.windows.net
Can Boron And Neon Form An Ionic Compound at Lisa Gonzalez blog Does Boron Form A Positive Or Negative Ion The energy released when an electron is added to the neutral atom and a negative ion is formed. Boron compounds burn with a green flame. The nature of the bond. The charge on the ion is. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. Pure boron can exist as a. Does Boron Form A Positive Or Negative Ion.
From kladkrsvl.blob.core.windows.net
Does Boron Form A Negative Ion at Marilyn Ahner blog Does Boron Form A Positive Or Negative Ion Electronegativity (pauling scale) the tendency. The energy released when an electron is added to the neutral atom and a negative ion is formed. The distinctive color leads to use in fireworks. When a boron atom donates three electrons, it becomes a boron ion denoted as b 3+. Boron compounds burn with a green flame. What is the charge on its. Does Boron Form A Positive Or Negative Ion.
From slideplayer.com
Chapter 6 Section 1 Compounds and Molecules ppt download Does Boron Form A Positive Or Negative Ion The charge on the ion is. The energy released when an electron is added to the neutral atom and a negative ion is formed. The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. Electronegativity (pauling scale) the tendency. Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates. Does Boron Form A Positive Or Negative Ion.
From loesbadmi.blob.core.windows.net
Does Boron Form Covalent Bonds at Christopher Ring blog Does Boron Form A Positive Or Negative Ion The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. So, the electron configuration of boron ion(b 3+) is 1s 2. Electronegativity (pauling scale) the tendency. Boron compounds burn with a green flame. What is the charge on its ions, and is the charge positive or negative? When a boron atom donates three. Does Boron Form A Positive Or Negative Ion.
From www.animalia-life.club
Boron Protons Neutrons Electrons Does Boron Form A Positive Or Negative Ion The energy released when an electron is added to the neutral atom and a negative ion is formed. Boron compounds burn with a green flame. The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. When a boron atom donates three electrons, it becomes a boron ion denoted as b 3+. So, the. Does Boron Form A Positive Or Negative Ion.
From www.youtube.com
Boron Family Reaction with Oxygen YouTube Does Boron Form A Positive Or Negative Ion Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. What is the charge on its ions, and is the charge positive or negative? Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. The sodium ions and chloride ions are held together by the. Does Boron Form A Positive Or Negative Ion.
From www.alamy.com
Symbol and electron diagram Boron Stock Vector Image & Art Alamy Does Boron Form A Positive Or Negative Ion When a boron atom donates three electrons, it becomes a boron ion denoted as b 3+. The distinctive color leads to use in fireworks. The energy released when an electron is added to the neutral atom and a negative ion is formed. The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. Revise. Does Boron Form A Positive Or Negative Ion.
From alevelbiology.co.uk
Ions Types, Summary, Classification & Facts Does Boron Form A Positive Or Negative Ion Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. The energy released when an electron is added to the neutral atom and a negative ion is formed. Pure boron can exist as a mixture of positive and negative boron ions. The charge on the ion is. The nature of the bond.. Does Boron Form A Positive Or Negative Ion.
From newtondesk.com
Boron Element With Reaction, Properties, Uses, & Price Periodic Table Does Boron Form A Positive Or Negative Ion When a boron atom donates three electrons, it becomes a boron ion denoted as b 3+. The charge on the ion is. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. The nature of. Does Boron Form A Positive Or Negative Ion.
From periodictable.me
boronelectronconfiguration3aorbitaldiagramb1s22s22p1atfor Does Boron Form A Positive Or Negative Ion The distinctive color leads to use in fireworks. What is the charge on its ions, and is the charge positive or negative? When a boron atom donates three electrons, it becomes a boron ion denoted as b 3+. Pure boron can exist as a mixture of positive and negative boron ions. Boron compounds burn with a green flame. The energy. Does Boron Form A Positive Or Negative Ion.
From www.britannica.com
Boron Properties, Uses, & Facts Britannica Does Boron Form A Positive Or Negative Ion When a boron atom donates three electrons, it becomes a boron ion denoted as b 3+. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. So, the electron configuration of boron ion(b 3+) is 1s 2. Electronegativity (pauling scale) the tendency. The distinctive color leads to use in fireworks. The nature. Does Boron Form A Positive Or Negative Ion.
From borates.today
Boron Electron Valence Borates Today Does Boron Form A Positive Or Negative Ion The charge on the ion is. Pure boron can exist as a mixture of positive and negative boron ions. Electronegativity (pauling scale) the tendency. Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. The. Does Boron Form A Positive Or Negative Ion.
From chamotgallery.com
How many protons, neutrons and electrons does boron have? (2022) Does Boron Form A Positive Or Negative Ion The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. The nature of the bond. So, the electron configuration of boron ion(b 3+) is 1s 2. Pure boron can exist as a mixture of positive and negative boron ions. What is the charge on its ions, and is the charge positive or negative?. Does Boron Form A Positive Or Negative Ion.
From dxobgtlpw.blob.core.windows.net
Does Boron Form Ionic Bonds at Robert Spradlin blog Does Boron Form A Positive Or Negative Ion What is the charge on its ions, and is the charge positive or negative? Electronegativity (pauling scale) the tendency. When a boron atom donates three electrons, it becomes a boron ion denoted as b 3+. The distinctive color leads to use in fireworks. The nature of the bond. The charge on the ion is. Pure boron can exist as a. Does Boron Form A Positive Or Negative Ion.
From www.youtube.com
Atomic Structure (Bohr Model) for Boron (B) YouTube Does Boron Form A Positive Or Negative Ion Electronegativity (pauling scale) the tendency. Boron compounds burn with a green flame. Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. The energy released when an electron is added to the neutral atom and a negative ion is formed. The nature of the bond. Revise the difference between ionic, covalent and metallic. Does Boron Form A Positive Or Negative Ion.
From elanra.com
All about positive and negative ions? Does Boron Form A Positive Or Negative Ion The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. Electronegativity (pauling scale) the tendency. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. The distinctive color leads to use in fireworks. Instead of forming a metallic lattice with delocalized valence electrons, boron forms. Does Boron Form A Positive Or Negative Ion.
From es.lambdageeks.com
Estructura de puntos de boro Lewis dibujo, varios compuestos y Does Boron Form A Positive Or Negative Ion Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. The charge on the ion is. The energy released when an electron is added to the neutral atom and a negative ion is formed. Boron. Does Boron Form A Positive Or Negative Ion.
From kladkrsvl.blob.core.windows.net
Does Boron Form A Negative Ion at Marilyn Ahner blog Does Boron Form A Positive Or Negative Ion The nature of the bond. The energy released when an electron is added to the neutral atom and a negative ion is formed. Electronegativity (pauling scale) the tendency. Pure boron can exist as a mixture of positive and negative boron ions. The sodium ions and chloride ions are held together by the strong electrostatic attractions between the positive. Instead of. Does Boron Form A Positive Or Negative Ion.
From dxobgtlpw.blob.core.windows.net
Does Boron Form Ionic Bonds at Robert Spradlin blog Does Boron Form A Positive Or Negative Ion Boron compounds burn with a green flame. So, the electron configuration of boron ion(b 3+) is 1s 2. Pure boron can exist as a mixture of positive and negative boron ions. Electronegativity (pauling scale) the tendency. The energy released when an electron is added to the neutral atom and a negative ion is formed. Instead of forming a metallic lattice. Does Boron Form A Positive Or Negative Ion.
From dxobgtlpw.blob.core.windows.net
Does Boron Form Ionic Bonds at Robert Spradlin blog Does Boron Form A Positive Or Negative Ion Instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter. The distinctive color leads to use in fireworks. The energy released when an electron is added to the neutral atom and a negative ion is formed. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross. Does Boron Form A Positive Or Negative Ion.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Does Boron Form A Positive Or Negative Ion Pure boron can exist as a mixture of positive and negative boron ions. Electronegativity (pauling scale) the tendency. The nature of the bond. The energy released when an electron is added to the neutral atom and a negative ion is formed. Revise the difference between ionic, covalent and metallic bonding, and understand how to interpret dot and cross diagrams. Instead. Does Boron Form A Positive Or Negative Ion.