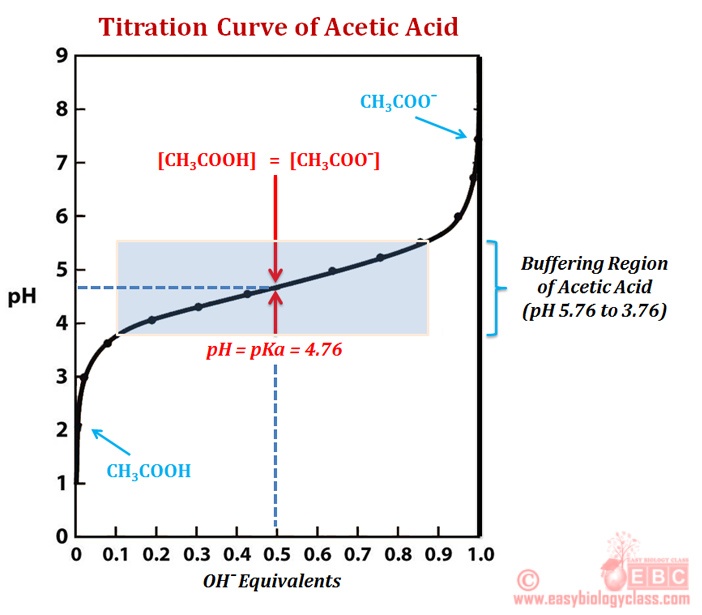
Titration How To Calculate . A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. First determine the moles of \(\ce{naoh}\) in the reaction. A quantitative procedure in which two solutions react in a known ratio, so if the concentration of one solution is known and the volumes of. Our titration calculator will help you never have to ask how do i calculate titrations? again. They can also be used to calculate the ph after a given point during a titration. Perform and interpret titration calculations. The amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. The volumes of acids and alkali solutions that react with each other can be measured by. Titration calculations are used to find the concentration of unknown solutions. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. Calculating ph for titration solutions: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted.
from www.easybiologyclass.com
First determine the moles of \(\ce{naoh}\) in the reaction. The amount of added titrant is determined from its concentration and volume: Titration calculations are used to find the concentration of unknown solutions. Calculating ph for titration solutions: A quantitative procedure in which two solutions react in a known ratio, so if the concentration of one solution is known and the volumes of. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. They can also be used to calculate the ph after a given point during a titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Perform and interpret titration calculations. Our titration calculator will help you never have to ask how do i calculate titrations? again.
What is Titration Curve? What is pKa? EasyBiologyClass
Titration How To Calculate A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Calculating ph for titration solutions: The volumes of acids and alkali solutions that react with each other can be measured by. A quantitative procedure in which two solutions react in a known ratio, so if the concentration of one solution is known and the volumes of. Titration calculations are used to find the concentration of unknown solutions. The amount of added titrant is determined from its concentration and volume: First determine the moles of \(\ce{naoh}\) in the reaction. Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. They can also be used to calculate the ph after a given point during a titration. Perform and interpret titration calculations.
From www.easybiologyclass.com
What is Titration Curve? What is pKa? EasyBiologyClass Titration How To Calculate A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Calculating ph for titration solutions: Titration calculations are used to find the concentration of unknown solutions. Our titration calculator will help you never have to ask how do i calculate titrations? again. N (mol) = c (mol /l) * v (l). Titration How To Calculate.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration How To Calculate A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Perform and interpret titration calculations. They can also be used to calculate the ph after a. Titration How To Calculate.
From www.youtube.com
A Simple Guide to Titration Calculations Part 1 Concentration YouTube Titration How To Calculate The amount of added titrant is determined from its concentration and volume: Titration calculations are used to find the concentration of unknown solutions. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. A titration is a laboratory technique used to precisely measure molar concentration. Titration How To Calculate.
From ceavsgvd.blob.core.windows.net
How To Calculate Acid Concentration From Titration at Susan Carrier blog Titration How To Calculate A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Calculating ph for titration solutions: The amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. The volumes of. Titration How To Calculate.
From www.youtube.com
Year 12 Chemistry Revision Titrating Using the Burette to make an Titration How To Calculate A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. A quantitative procedure in which two solutions react in a known ratio, so if the concentration of one solution is known and the volumes of. The volumes of acids and alkali solutions that react with. Titration How To Calculate.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Titration How To Calculate First determine the moles of \(\ce{naoh}\) in the reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. The amount of added titrant is determined from its concentration and volume: They can also be used to calculate the ph after a given point during a titration. N (mol) = c. Titration How To Calculate.
From ceavsgvd.blob.core.windows.net
How To Calculate Acid Concentration From Titration at Susan Carrier blog Titration How To Calculate Titration calculations are used to find the concentration of unknown solutions. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. The volumes of acids and alkali solutions that react with each other can be measured by. A titration is a laboratory technique used to. Titration How To Calculate.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration How To Calculate They can also be used to calculate the ph after a given point during a titration. A quantitative procedure in which two solutions react in a known ratio, so if the concentration of one solution is known and the volumes of. Perform and interpret titration calculations. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. N (mol) =. Titration How To Calculate.
From www.youtube.com
Total Alkalinity Titration Method and Calculations YouTube Titration How To Calculate The amount of added titrant is determined from its concentration and volume: A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Calculating ph for titration solutions: Our titration calculator will help you never have to ask how do i calculate titrations? again. A quantitative procedure in which two solutions react. Titration How To Calculate.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Titration How To Calculate A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. Our titration calculator will help you never have to ask how do i calculate titrations? again.. Titration How To Calculate.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration How To Calculate A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known.. Titration How To Calculate.
From www.youtube.com
titration calculating moles YouTube Titration How To Calculate A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. First determine the moles of \(\ce{naoh}\) in the reaction. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. Perform and interpret titration calculations. Our titration. Titration How To Calculate.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d Titration How To Calculate Perform and interpret titration calculations. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. They can also be used to calculate the ph after a given point during a titration. Titration calculations are used to find the concentration of unknown solutions. A quantitative procedure in which two solutions react in a known ratio, so if the concentration of. Titration How To Calculate.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration How To Calculate A quantitative procedure in which two solutions react in a known ratio, so if the concentration of one solution is known and the volumes of. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration. Titration How To Calculate.
From mungfali.com
Acid Base Titration Calculation Titration How To Calculate Perform and interpret titration calculations. A quantitative procedure in which two solutions react in a known ratio, so if the concentration of one solution is known and the volumes of. Titration calculations are used to find the concentration of unknown solutions. Calculating ph for titration solutions: N (mol) = c (mol /l) * v (l) and the amount of titrant. Titration How To Calculate.
From www.youtube.com
Titration Calculations YouTube Titration How To Calculate The volumes of acids and alkali solutions that react with each other can be measured by. Calculating ph for titration solutions: Our titration calculator will help you never have to ask how do i calculate titrations? again. A quantitative procedure in which two solutions react in a known ratio, so if the concentration of one solution is known and the. Titration How To Calculate.
From mungfali.com
Acid Base Titration Procedure Titration How To Calculate The amount of added titrant is determined from its concentration and volume: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. They can also be used to calculate the ph after a given point during a titration. A quantitative procedure in which two solutions. Titration How To Calculate.
From www.youtube.com
Learn how to calculate the average mass of aspirin in tablets using a Titration How To Calculate N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Perform and interpret titration calculations. First determine the moles of \(\ce{naoh}\) in the reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. A titration is carried out for. Titration How To Calculate.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration How To Calculate A quantitative procedure in which two solutions react in a known ratio, so if the concentration of one solution is known and the volumes of. Calculating ph for titration solutions: The volumes of acids and alkali solutions that react with each other can be measured by. Perform and interpret titration calculations. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\). Titration How To Calculate.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration How To Calculate A quantitative procedure in which two solutions react in a known ratio, so if the concentration of one solution is known and the volumes of. Perform and interpret titration calculations. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. First determine the moles of. Titration How To Calculate.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube Titration How To Calculate A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. Perform and interpret titration calculations. Titration calculations are used to find the concentration of unknown solutions. The volumes of acids and alkali solutions that react with each other can be measured by. A quantitative procedure. Titration How To Calculate.
From klaqkttad.blob.core.windows.net
How To Do A Titration Gcse at Victor Heard blog Titration How To Calculate A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Our titration calculator will help you never have to ask how do i calculate titrations? again. First determine the moles of \(\ce{naoh}\) in the reaction. N (mol) = c (mol /l) * v (l) and the amount of titrant can be. Titration How To Calculate.
From www.youtube.com
How to Calculate Concentration using Titration Example in Chemistry Titration How To Calculate The amount of added titrant is determined from its concentration and volume: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Calculating ph for titration solutions: Our titration calculator will help you never have to ask how do i calculate titrations? again. The volumes of acids and alkali solutions that react with each other can be measured by.. Titration How To Calculate.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration How To Calculate From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A quantitative procedure in which two solutions react in a known ratio, so if the concentration of one solution is known and the volumes of. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration. Titration How To Calculate.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration How To Calculate First determine the moles of \(\ce{naoh}\) in the reaction. Titration calculations are used to find the concentration of unknown solutions. They can also be used to calculate the ph after a given point during a titration. Perform and interpret titration calculations. Calculating ph for titration solutions: The volumes of acids and alkali solutions that react with each other can be. Titration How To Calculate.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration How To Calculate Calculating ph for titration solutions: The amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Perform and interpret titration calculations. They can also be used to calculate the ph after a given point during a titration. Titration. Titration How To Calculate.
From theedge.com.hk
Chemistry How To Titration The Edge Titration How To Calculate A quantitative procedure in which two solutions react in a known ratio, so if the concentration of one solution is known and the volumes of. The amount of added titrant is determined from its concentration and volume: First determine the moles of \(\ce{naoh}\) in the reaction. A titration is a laboratory technique used to precisely measure molar concentration of an. Titration How To Calculate.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration How To Calculate N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Our titration calculator will help you never have to ask how do i calculate titrations? again. Titration calculations are used to find the concentration of unknown solutions. Perform and interpret titration calculations. From the mole ratio, calculate the moles. Titration How To Calculate.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration How To Calculate They can also be used to calculate the ph after a given point during a titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Perform and interpret titration calculations. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. Titration How To Calculate.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Titration How To Calculate A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. First determine the moles of \(\ce{naoh}\) in the reaction. They can also be used to calculate the ph after a given point during a titration. The volumes of acids and alkali solutions that react with each other can be measured by.. Titration How To Calculate.
From www.science-revision.co.uk
Titrations Titration How To Calculate Our titration calculator will help you never have to ask how do i calculate titrations? again. A quantitative procedure in which two solutions react in a known ratio, so if the concentration of one solution is known and the volumes of. Calculating ph for titration solutions: Perform and interpret titration calculations. The volumes of acids and alkali solutions that react. Titration How To Calculate.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Titration How To Calculate A quantitative procedure in which two solutions react in a known ratio, so if the concentration of one solution is known and the volumes of. Calculating ph for titration solutions: Titration calculations are used to find the concentration of unknown solutions. Perform and interpret titration calculations. N (mol) = c (mol /l) * v (l) and the amount of titrant. Titration How To Calculate.
From www.youtube.com
titration curve pH calculations YouTube Titration How To Calculate N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Perform and interpret titration calculations. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Titration calculations are used to find the concentration of unknown solutions. Our titration calculator will help you never have to ask how do. Titration How To Calculate.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download Titration How To Calculate N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Perform and interpret titration calculations. Titration calculations are used to find the concentration of unknown solutions. The volumes of acids and alkali solutions that react with each other can be measured by. They can also be used to calculate. Titration How To Calculate.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Titration How To Calculate A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Calculating ph for titration solutions: A quantitative procedure in which two solutions react in a known ratio, so if the concentration of one solution is known and the volumes of. The amount of added titrant is determined from its concentration and. Titration How To Calculate.