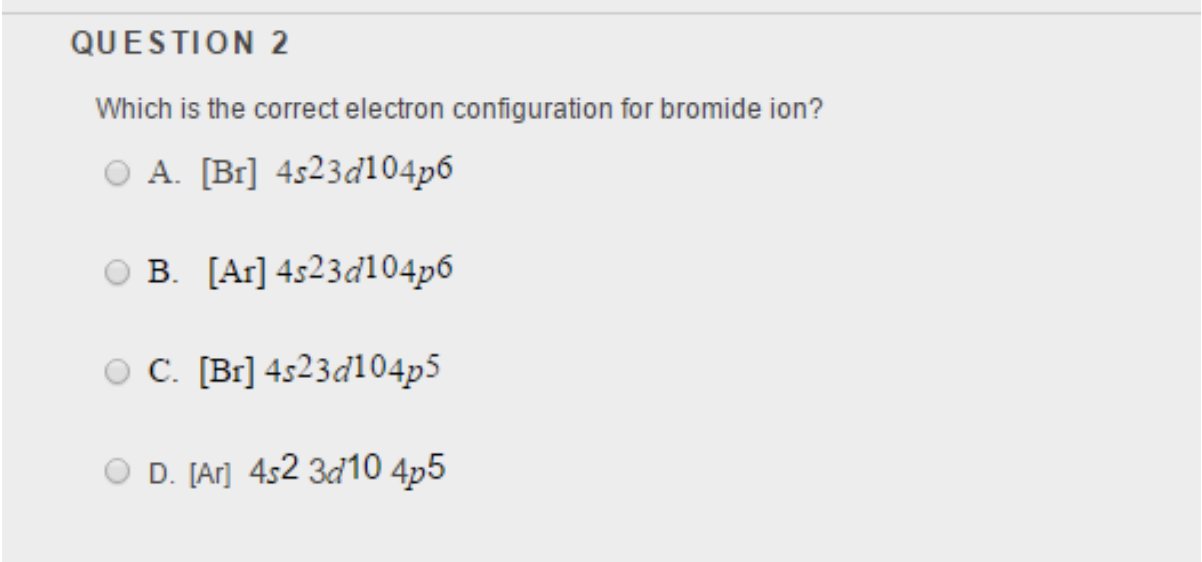
Bromide Anion Electron Configuration . The following table lists the ionization energies ie (ionization potentials); We add electrons to fill the. How do the electron configurations of transition metals differ from those of other elements? Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. The bromide ion has the same electron configuration as a neutral bromine. The ie is the energy required in electron volt (ev) per atom to. The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The electron configuration for the magnesium cation is:
from brodskymuchey.blogspot.com
The electron configuration for the magnesium cation is: We add electrons to fill the. Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The ie is the energy required in electron volt (ev) per atom to. The bromide ion has the same electron configuration as a neutral bromine. The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. How do the electron configurations of transition metals differ from those of other elements? The following table lists the ionization energies ie (ionization potentials);
Same Electron Configuration as the Bromide Ion Br Brodsky Muchey
Bromide Anion Electron Configuration The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The bromide ion has the same electron configuration as a neutral bromine. The electron configuration for the magnesium cation is: The following table lists the ionization energies ie (ionization potentials); The ie is the energy required in electron volt (ev) per atom to. We add electrons to fill the. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. How do the electron configurations of transition metals differ from those of other elements?
From www.youtube.com
Electron Configurations of Anions YouTube Bromide Anion Electron Configuration The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. We add electrons to fill the. The ie is the energy required in electron volt (ev) per atom to. How do the electron configurations of transition metals differ from those of other elements? The bromide ion has the same electron configuration as a neutral. Bromide Anion Electron Configuration.
From www.chemistrylearner.com
Sodium Bromate Facts, Formula, Properties, Uses, Safety Data Bromide Anion Electron Configuration The following table lists the ionization energies ie (ionization potentials); Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The bromide ion has the same electron configuration as a neutral bromine. The electron configuration for the magnesium cation is: How do the electron configurations of transition metals differ from those of other elements? The. Bromide Anion Electron Configuration.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromide Anion Electron Configuration The bromide ion has the same electron configuration as a neutral bromine. The ie is the energy required in electron volt (ev) per atom to. The electron configuration for the magnesium cation is: We add electrons to fill the. Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. How do the electron configurations of. Bromide Anion Electron Configuration.
From sielc.com
Vinyl bromide SIELC Technologies Bromide Anion Electron Configuration We add electrons to fill the. How do the electron configurations of transition metals differ from those of other elements? The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. The bromide ion has the same electron configuration as a neutral bromine. The electron configuration for the magnesium cation is: The electronic configuration of anions is assigned by. Bromide Anion Electron Configuration.
From byjus.com
Write the molecular formulae for the compound Copper II Bromide Bromide Anion Electron Configuration The bromide ion has the same electron configuration as a neutral bromine. The following table lists the ionization energies ie (ionization potentials); Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. We add electrons to fill the. The. Bromide Anion Electron Configuration.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromide Anion Electron Configuration How do the electron configurations of transition metals differ from those of other elements? The following table lists the ionization energies ie (ionization potentials); The bromide ion has the same electron configuration as a neutral bromine. We add electrons to fill the. The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The electron. Bromide Anion Electron Configuration.
From www.firsthope.co.in
Methantheline Bromide Chemical Structure, Mechanism of Action, Uses Bromide Anion Electron Configuration The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. How do the electron configurations of transition metals differ from those of other elements? The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The ie is. Bromide Anion Electron Configuration.
From pnghut.com
Electron Configuration Bromine Chemical Element Shell Bohr Model Bromide Anion Electron Configuration The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. How do the electron configurations of transition metals differ from those of other elements? The bromide ion has the same electron configuration as a neutral bromine. The electron configuration for the magnesium cation is: We add electrons to fill the. The following table lists the ionization energies ie. Bromide Anion Electron Configuration.
From www.vectorstock.com
Ch3br methyl bromide molecule Royalty Free Vector Image Bromide Anion Electron Configuration The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The following table lists the ionization energies ie (ionization potentials); The ie is the energy required in electron volt (ev) per atom to. Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. We add electrons to fill the.. Bromide Anion Electron Configuration.
From favpng.com
Tin Bromide Lewis Structure Tin(IV) Oxide Structural Formula, PNG Bromide Anion Electron Configuration The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. The following table lists the ionization energies ie (ionization potentials); We add electrons to fill the. How do the electron configurations of transition metals differ from those of other elements? The ie is the energy required in electron volt (ev) per atom to. The bromide ion has the. Bromide Anion Electron Configuration.
From brodskymuchey.blogspot.com
Same Electron Configuration as the Bromide Ion Br Brodsky Muchey Bromide Anion Electron Configuration Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. We add electrons to fill the. The following table lists the ionization energies ie (ionization potentials); The bromide ion has the same electron configuration as a neutral bromine. The electron configuration for the. Bromide Anion Electron Configuration.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromide Anion Electron Configuration The bromide ion has the same electron configuration as a neutral bromine. Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The ie is the energy required in electron volt (ev) per atom to. How do the electron. Bromide Anion Electron Configuration.
From www.shutterstock.com
Sodium Bromide Properties Chemical Compound Structure Stock Vector Bromide Anion Electron Configuration We add electrons to fill the. The bromide ion has the same electron configuration as a neutral bromine. How do the electron configurations of transition metals differ from those of other elements? Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The following table lists the ionization energies ie (ionization potentials); The electron configuration. Bromide Anion Electron Configuration.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Bromide Anion Electron Configuration Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The bromide ion has the same electron configuration as a neutral bromine. How do the electron configurations of transition metals differ from those of other elements? We add electrons to fill the. The ie is the energy required in electron volt (ev) per atom to.. Bromide Anion Electron Configuration.
From general.chemistrysteps.com
Electron Configurations Chemistry Steps Bromide Anion Electron Configuration Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The following table lists the ionization energies ie (ionization potentials); The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. We add electrons to fill the. How do the electron configurations of transition metals differ from those of other. Bromide Anion Electron Configuration.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Bromide Anion Electron Configuration The following table lists the ionization energies ie (ionization potentials); We add electrons to fill the. The electron configuration for the magnesium cation is: How do the electron configurations of transition metals differ from those of other elements? The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The bromide ion has the same. Bromide Anion Electron Configuration.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Bromide Anion Electron Configuration The following table lists the ionization energies ie (ionization potentials); The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. The bromide ion has the same electron configuration as a neutral bromine. The electron configuration for the magnesium cation is: Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. We add electrons. Bromide Anion Electron Configuration.
From slideplayer.com
Ionic Bonding and Ionic Compounds ppt download Bromide Anion Electron Configuration The bromide ion has the same electron configuration as a neutral bromine. How do the electron configurations of transition metals differ from those of other elements? The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. The electron configuration for the magnesium cation is: The electronic configuration of anions is assigned by adding electrons according to aufbau's building. Bromide Anion Electron Configuration.
From testbook.com
Lithium Bromide Learn Structure, Properties, Preparation & Uses. Bromide Anion Electron Configuration The ie is the energy required in electron volt (ev) per atom to. The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. The electron configuration for the magnesium cation is: The following table lists the ionization energies ie (ionization potentials); Most. Bromide Anion Electron Configuration.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Bromide Anion Electron Configuration We add electrons to fill the. How do the electron configurations of transition metals differ from those of other elements? The following table lists the ionization energies ie (ionization potentials); Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The electronic configuration of anions is assigned by adding electrons according to aufbau's building up. Bromide Anion Electron Configuration.
From www.alamyimages.fr
Schéma d'électrons et de symbole pour le brome illustration Image Bromide Anion Electron Configuration The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The following table lists the ionization energies ie (ionization potentials); The electron configuration for the magnesium cation is: How do the electron configurations of transition metals differ from those of other elements? The ie is the energy required in electron volt (ev) per atom. Bromide Anion Electron Configuration.
From ar.inspiredpencil.com
Electron Configuration Of Bromine Bromide Anion Electron Configuration The ie is the energy required in electron volt (ev) per atom to. Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The following table lists the ionization energies ie (ionization potentials); The bromide ion has the same electron configuration as a neutral bromine. The electron configuration for the magnesium cation is: The electron. Bromide Anion Electron Configuration.
From byjus.com
Briefly explain the process of electrolysis of molten Lead Bromide Bromide Anion Electron Configuration The ie is the energy required in electron volt (ev) per atom to. The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The electron configuration for the magnesium cation is: The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. Most monatomic anions form when a neutral nonmetal atom gains enough. Bromide Anion Electron Configuration.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Bromide Anion Electron Configuration How do the electron configurations of transition metals differ from those of other elements? The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. The ie is the energy required in electron volt (ev) per atom to. The bromide ion has the same electron configuration as a neutral bromine. The electronic configuration of anions is assigned by adding. Bromide Anion Electron Configuration.
From www.numerade.com
There are four alkyl bromides with the formula C 4 H9 Br. Write their Bromide Anion Electron Configuration The bromide ion has the same electron configuration as a neutral bromine. The electron configuration for the magnesium cation is: The following table lists the ionization energies ie (ionization potentials); How do the electron configurations of transition metals differ from those of other elements? The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle.. Bromide Anion Electron Configuration.
From www.macsenlab.com
Pinaverium Bromide API 53251948 Manufacturer & Supplier Bromide Anion Electron Configuration Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The ie is the energy required in electron volt (ev) per atom to. We add electrons to fill the. The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The electron configuration of the bromide ion is [ar] 4s2. Bromide Anion Electron Configuration.
From www.alamy.com
Cetrimonium bromide antiseptic surfactant molecule. Skeletal formula Bromide Anion Electron Configuration The ie is the energy required in electron volt (ev) per atom to. The electron configuration for the magnesium cation is: The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. We add electrons to fill the. The bromide. Bromide Anion Electron Configuration.
From www.researchgate.net
Top Chemical structures of CPEs and salts tetraethylammonium bromide Bromide Anion Electron Configuration The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. The following table lists the ionization energies ie (ionization potentials); The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The ie is the energy required in electron volt (ev) per atom to. The electron configuration for the magnesium cation is: The. Bromide Anion Electron Configuration.
From us.metoree.com
41 Ethyl Bromide Manufacturers in 2024 Metoree Bromide Anion Electron Configuration The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The ie is the energy required in electron volt (ev) per atom to. We add electrons to. Bromide Anion Electron Configuration.
From sielc.com
Methyl bromide SIELC Technologies Bromide Anion Electron Configuration The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. The electron configuration for the magnesium cation is: The bromide ion has the same electron configuration as a neutral bromine. The following table lists the ionization energies ie (ionization potentials); The ie. Bromide Anion Electron Configuration.
From mavericksrpatterson.blogspot.com
Chemical Formula of Bromide MavericksrPatterson Bromide Anion Electron Configuration The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. How do the electron configurations of transition metals differ from those of other elements? The ie is the energy required in electron volt (ev) per atom to. The following. Bromide Anion Electron Configuration.
From www.rpicorp.com
E718005.0 Ethidium Bromide, Powder, 5 Grams Bromide Anion Electron Configuration Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The bromide ion has the same electron configuration as a neutral bromine. The ie is the energy required in electron volt (ev) per atom to. How do the electron configurations of transition metals differ from those of other elements? We add electrons to fill the.. Bromide Anion Electron Configuration.
From www.alamy.com
Gel electrophoresis ethidium bromide hires stock photography and Bromide Anion Electron Configuration How do the electron configurations of transition metals differ from those of other elements? The ie is the energy required in electron volt (ev) per atom to. The electronic configuration of anions is assigned by adding electrons according to aufbau's building up principle. The bromide ion has the same electron configuration as a neutral bromine. The electron configuration for the. Bromide Anion Electron Configuration.
From hamptonresearch.com
Hampton Research Bromide Anion Electron Configuration Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The electron configuration of the bromide ion is [ar] 4s2 3d10 4p6. The bromide ion has the same electron configuration as a neutral bromine. The ie is the energy required in electron volt (ev) per atom to. The electronic configuration of anions is assigned by. Bromide Anion Electron Configuration.
From brainly.com
Which particle represents the size of the bromide ion compared to the Bromide Anion Electron Configuration We add electrons to fill the. The bromide ion has the same electron configuration as a neutral bromine. The electron configuration for the magnesium cation is: The ie is the energy required in electron volt (ev) per atom to. Most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill. The electronic configuration of anions is. Bromide Anion Electron Configuration.