Dilution Factor Formula Concentration . learn the formula, components, and applications of dilution factor in scientific research. For example, adding more water to sugar syrup leads to decrease in. — concentration is the reverse of dilution. Find out how to avoid common. — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing. — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a. — learn how to calculate the dilution factor, the ratio of the stock solution to the dilutant or the total solution, using a simple formula. Enter the volume of stock solution and dilutant to get the dilution factor as a. learn what dilution factor is and how to calculate it for any dilution you prepare. — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted.
from www.slideshare.net
learn what dilution factor is and how to calculate it for any dilution you prepare. Enter the volume of stock solution and dilutant to get the dilution factor as a. — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. — concentration is the reverse of dilution. For example, adding more water to sugar syrup leads to decrease in. Find out how to avoid common. learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a. — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing. learn the formula, components, and applications of dilution factor in scientific research.
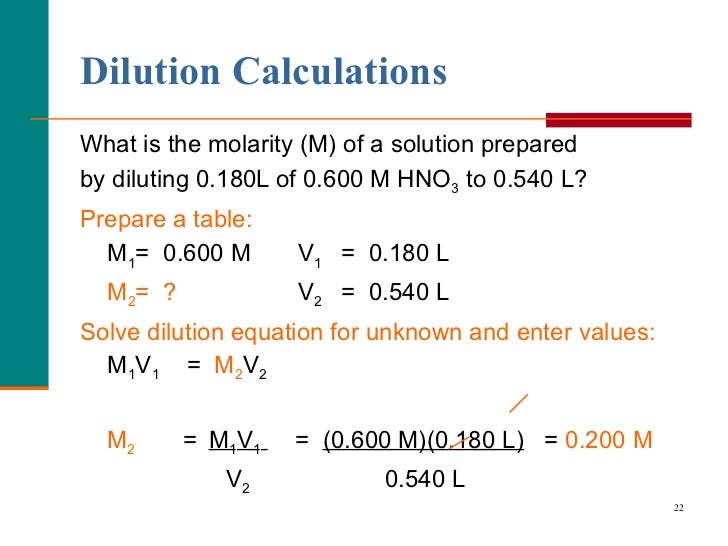
Molarity and dilution
Dilution Factor Formula Concentration — concentration is the reverse of dilution. Find out how to avoid common. learn the formula, components, and applications of dilution factor in scientific research. — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing. For example, adding more water to sugar syrup leads to decrease in. learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a. learn what dilution factor is and how to calculate it for any dilution you prepare. — concentration is the reverse of dilution. Enter the volume of stock solution and dilutant to get the dilution factor as a. — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. — learn how to calculate the dilution factor, the ratio of the stock solution to the dilutant or the total solution, using a simple formula.
From www.youtube.com
How solve dilution and concentration calculation problems YouTube Dilution Factor Formula Concentration For example, adding more water to sugar syrup leads to decrease in. learn the formula, components, and applications of dilution factor in scientific research. — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. Find out how to avoid common. — learn how to calculate. Dilution Factor Formula Concentration.
From www.freepik.com
Premium Vector Dilution concentration formula science vector Dilution Factor Formula Concentration learn the formula, components, and applications of dilution factor in scientific research. learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a. — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. Find. Dilution Factor Formula Concentration.
From www.pdfprof.com
how to calculate dilution factor Dilution Factor Formula Concentration Find out how to avoid common. — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing. learn the formula, components, and applications of dilution factor in scientific research. Enter the volume of stock solution and dilutant to get the dilution factor as a.. Dilution Factor Formula Concentration.
From lasopamail784.weebly.com
Calculate Dilution Factor lasopamail Dilution Factor Formula Concentration For example, adding more water to sugar syrup leads to decrease in. — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. Find out how to avoid common. — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume. Dilution Factor Formula Concentration.
From quizdemurrages.z21.web.core.windows.net
Calculate Dilution Percent Dilution Factor Formula Concentration — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. Enter the volume of stock solution and dilutant to get the dilution factor as a. — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution. Dilution Factor Formula Concentration.
From www.freepik.com
Premium Vector Dilution factor formula science vector illustration Dilution Factor Formula Concentration — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. — concentration is the reverse of dilution. — learn how to calculate the dilution factor, the ratio of the stock solution to the dilutant or the total solution, using a simple formula. — as you. Dilution Factor Formula Concentration.
From www.pdfprof.com
how to calculate dilution factor Dilution Factor Formula Concentration — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. For example, adding more water to sugar syrup leads to decrease in. — learn. Dilution Factor Formula Concentration.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Dilution Factor Formula Concentration — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing. learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a. learn the formula, components, and applications of dilution factor in. Dilution Factor Formula Concentration.
From www.youtube.com
Calculating the concentration of a solution using a dilution factor Dilution Factor Formula Concentration Find out how to avoid common. Enter the volume of stock solution and dilutant to get the dilution factor as a. — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. — learn how to calculate the dilution factor, the ratio of the stock solution to the. Dilution Factor Formula Concentration.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Factor Formula Concentration — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing. — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. learn what dilution factor is and how to calculate it. Dilution Factor Formula Concentration.
From www.hemocytometer.org
Using the dilution factor to calculate dilutions • Hemocytometer Dilution Factor Formula Concentration Find out how to avoid common. Enter the volume of stock solution and dilutant to get the dilution factor as a. — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing. — concentration is the reverse of dilution. — learn how to. Dilution Factor Formula Concentration.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Factor Formula Concentration — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing. — concentration is the reverse of dilution. — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. learn the. Dilution Factor Formula Concentration.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration Dilution Factor Formula Concentration — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. For example, adding more water to sugar syrup leads to decrease in. Find out how to avoid common. learn the formula, components, and applications of dilution factor in scientific research. — concentration is the reverse of. Dilution Factor Formula Concentration.
From www.youtube.com
Chem 11 Unit 5 Dilution Calculations Solving for Final Concentration Dilution Factor Formula Concentration Enter the volume of stock solution and dilutant to get the dilution factor as a. learn what dilution factor is and how to calculate it for any dilution you prepare. — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. learn how to calculate the. Dilution Factor Formula Concentration.
From www.slideserve.com
PPT Concentration PowerPoint Presentation, free download ID2186169 Dilution Factor Formula Concentration — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. Find out how to avoid common. — learn how to calculate the dilution factor,. Dilution Factor Formula Concentration.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution Factor Formula Concentration Find out how to avoid common. — concentration is the reverse of dilution. — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing. learn the formula, components, and applications of dilution factor in scientific research. — as you know, the concentration. Dilution Factor Formula Concentration.
From sciencestruck.com
Here's How to Calculate Dilution Factor from Given Concentration Dilution Factor Formula Concentration Find out how to avoid common. learn the formula, components, and applications of dilution factor in scientific research. For example, adding more water to sugar syrup leads to decrease in. learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a. — learn how to calculate. Dilution Factor Formula Concentration.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution Factor Formula Concentration Enter the volume of stock solution and dilutant to get the dilution factor as a. — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. — concentration is the reverse of dilution. Find out how to avoid common. — learn how to use the dilution. Dilution Factor Formula Concentration.
From onthewebvil.weebly.com
Formula for concentration dilution onthewebvil Dilution Factor Formula Concentration learn the formula, components, and applications of dilution factor in scientific research. Find out how to avoid common. Enter the volume of stock solution and dilutant to get the dilution factor as a. — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing.. Dilution Factor Formula Concentration.
From www.youtube.com
Calculate dilution factor and concentration when diluting liquid and Dilution Factor Formula Concentration — concentration is the reverse of dilution. — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing. — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. learn what dilution. Dilution Factor Formula Concentration.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Dilution Factor Formula Concentration — concentration is the reverse of dilution. For example, adding more water to sugar syrup leads to decrease in. learn what dilution factor is and how to calculate it for any dilution you prepare. learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a. Enter. Dilution Factor Formula Concentration.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Dilution Factor Formula Concentration — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing. — learn how to calculate the dilution factor, the ratio. Dilution Factor Formula Concentration.
From www.slideserve.com
PPT Dilution, Concentration and Alligation PowerPoint Presentation Dilution Factor Formula Concentration learn the formula, components, and applications of dilution factor in scientific research. Enter the volume of stock solution and dilutant to get the dilution factor as a. — concentration is the reverse of dilution. — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding. Dilution Factor Formula Concentration.
From www.thetechedvocate.org
How to Calculate the Dilution Factor The Tech Edvocate Dilution Factor Formula Concentration — concentration is the reverse of dilution. — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a. Enter the volume of stock solution and. Dilution Factor Formula Concentration.
From www.youtube.com
Dilution concentration calculation YouTube Dilution Factor Formula Concentration — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. learn how to calculate the dilution factor, a ratio or exponent that describes the. Dilution Factor Formula Concentration.
From socratic.org
How can I calculate the dilution factor using concentration? Socratic Dilution Factor Formula Concentration For example, adding more water to sugar syrup leads to decrease in. — concentration is the reverse of dilution. learn the formula, components, and applications of dilution factor in scientific research. — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. Enter the volume of stock. Dilution Factor Formula Concentration.
From www.youtube.com
How to Calculate Dilution Factor YouTube Dilution Factor Formula Concentration — concentration is the reverse of dilution. — learn how to calculate the dilution factor, the ratio of the stock solution to the dilutant or the total solution, using a simple formula. For example, adding more water to sugar syrup leads to decrease in. Find out how to avoid common. — learn how to use the dilution. Dilution Factor Formula Concentration.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Factor Formula Concentration Find out how to avoid common. Enter the volume of stock solution and dilutant to get the dilution factor as a. learn the formula, components, and applications of dilution factor in scientific research. — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing.. Dilution Factor Formula Concentration.
From www.slideshare.net
Molarity and dilution Dilution Factor Formula Concentration — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution. — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. Find out how to avoid common. learn the formula, components, and applications of dilution. Dilution Factor Formula Concentration.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Factor Formula Concentration Enter the volume of stock solution and dilutant to get the dilution factor as a. — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. — as you know, the concentration of a solution is defined as the number of moles of solute per liter of solution.. Dilution Factor Formula Concentration.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Factor Formula Concentration — concentration is the reverse of dilution. Find out how to avoid common. — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. learn what dilution factor is and how to calculate it for any dilution you prepare. — learn how to use the dilution. Dilution Factor Formula Concentration.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution Factor Formula Concentration For example, adding more water to sugar syrup leads to decrease in. learn the formula, components, and applications of dilution factor in scientific research. Enter the volume of stock solution and dilutant to get the dilution factor as a. Find out how to avoid common. learn how to calculate the dilution factor, a ratio or exponent that describes. Dilution Factor Formula Concentration.
From www.slideshare.net
Molarity and dilution Dilution Factor Formula Concentration — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. — learn how to calculate the dilution factor, the ratio of the stock solution to the dilutant or the total solution, using a simple formula. — as you know, the concentration of a solution is defined. Dilution Factor Formula Concentration.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Factor Formula Concentration — learn how to calculate the dilution factor, the ratio of the stock solution to the dilutant or the total solution, using a simple formula. learn what dilution factor is and how to calculate it for any dilution you prepare. — learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or. Dilution Factor Formula Concentration.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Factor Formula Concentration — learn how to use the dilution equation (c1v1 = c2v2) to calculate the final concentration or volume of a diluted. Enter the volume of stock solution and dilutant to get the dilution factor as a. — learn how to calculate the dilution factor, the ratio of the stock solution to the dilutant or the total solution, using. Dilution Factor Formula Concentration.