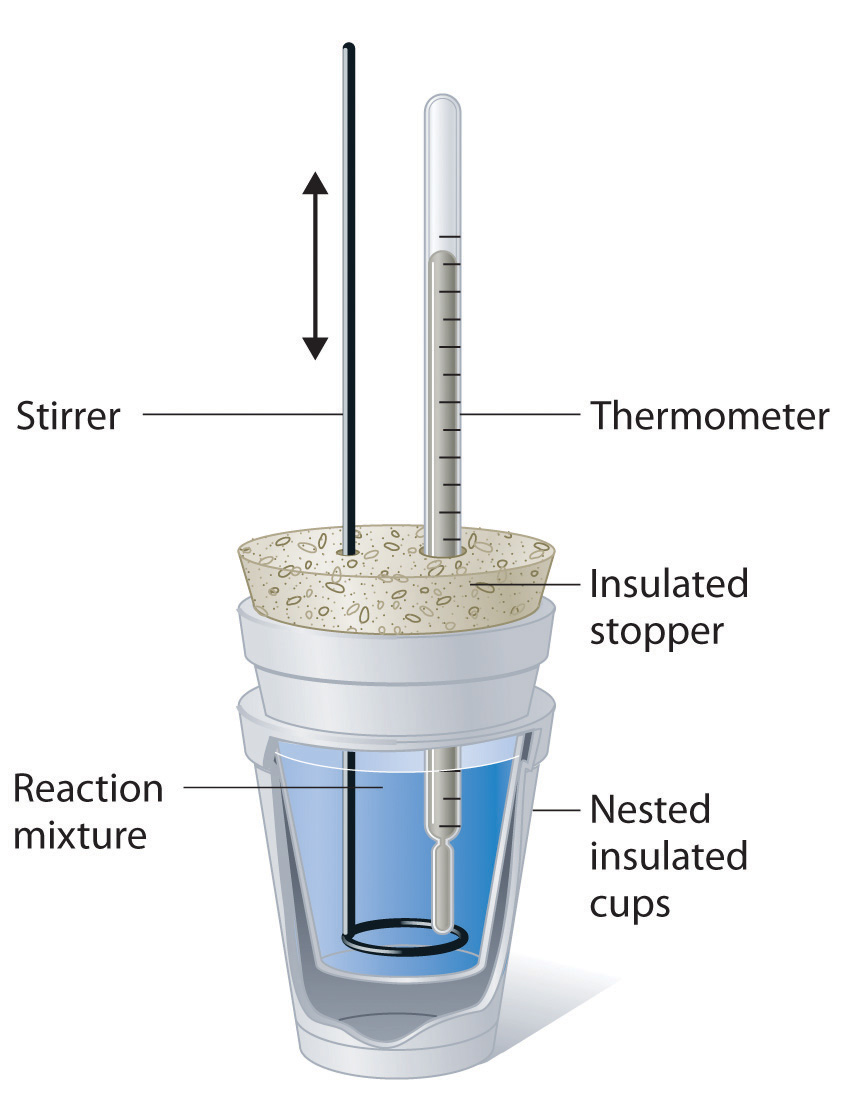
Calorimeter Experiment Calculations . To apply these \( \delta h\) values in a. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. Q = m × c × δt. to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. solve problems involving heat transfer. calorimetry is used to measure amounts of heat transferred to or from a substance. We have seen in previous chapters that energy is one of the fundamental concepts of physics. M is the mass of the. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. We use capital cc to represent the heat. It can analyze the heat exchange between up to 3 objects. the calorimetry calculator can help you solve complex calorimetry problems. To do so, the heat is exchanged with a calibrated. The equation to calculate enthalpy changes from temperature changes is:
from users.highland.edu
The equation to calculate enthalpy changes from temperature changes is: solve problems involving heat transfer. It can analyze the heat exchange between up to 3 objects. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. Q is the heat energy. the calorimetry calculator can help you solve complex calorimetry problems. M is the mass of the. To do so, the heat is exchanged with a calibrated. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. We have seen in previous chapters that energy is one of the fundamental concepts of physics.
Calorimetry
Calorimeter Experiment Calculations We use capital cc to represent the heat. We use capital cc to represent the heat. The equation to calculate enthalpy changes from temperature changes is: To do so, the heat is exchanged with a calibrated. Q = m × c × δt. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Q is the heat energy. the calorimetry calculator can help you solve complex calorimetry problems. solve problems involving heat transfer. To apply these \( \delta h\) values in a. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. calorimetry is used to measure amounts of heat transferred to or from a substance. We have seen in previous chapters that energy is one of the fundamental concepts of physics. M is the mass of the. It can analyze the heat exchange between up to 3 objects. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance.
From answermagicmatney.z21.web.core.windows.net
Coffee Cup Calorimetry Experiment Calorimeter Experiment Calculations the calorimetry calculator can help you solve complex calorimetry problems. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. It can analyze the heat exchange between up to 3 objects. to make sure you get accurate results you need to. Calorimeter Experiment Calculations.
From www.chegg.com
Solved Name Section Experiment 14 Data And Calculations Calorimeter Experiment Calculations To apply these \( \delta h\) values in a. The equation to calculate enthalpy changes from temperature changes is: in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. It can analyze the heat exchange between up to 3 objects. Q is the. Calorimeter Experiment Calculations.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Calorimeter Experiment Calculations M is the mass of the. to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. solve problems involving heat transfer. We have seen in previous chapters that energy is one of the fundamental concepts of physics. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of. Calorimeter Experiment Calculations.
From www.tessshebaylo.com
Equation For Determining Calorimetry Tessshebaylo Calorimeter Experiment Calculations To apply these \( \delta h\) values in a. The equation to calculate enthalpy changes from temperature changes is: It can analyze the heat exchange between up to 3 objects. To do so, the heat is exchanged with a calibrated. the calorimetry calculator can help you solve complex calorimetry problems. We have seen in previous chapters that energy is. Calorimeter Experiment Calculations.
From www.slideserve.com
PPT Energy in food PowerPoint Presentation, free download ID5830775 Calorimeter Experiment Calculations Q is the heat energy. It can analyze the heat exchange between up to 3 objects. We have seen in previous chapters that energy is one of the fundamental concepts of physics. To apply these \( \delta h\) values in a. We use capital cc to represent the heat. Q = m × c × δt. experiment 6 ∙. Calorimeter Experiment Calculations.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimeter Experiment Calculations Q is the heat energy. to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. We have seen in previous chapters that energy is one of the fundamental concepts of. Calorimeter Experiment Calculations.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimeter Experiment Calculations We use capital cc to represent the heat. To do so, the heat is exchanged with a calibrated. It can analyze the heat exchange between up to 3 objects. M is the mass of the. The equation to calculate enthalpy changes from temperature changes is: experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water. Calorimeter Experiment Calculations.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimeter Experiment Calculations To apply these \( \delta h\) values in a. We use capital cc to represent the heat. It can analyze the heat exchange between up to 3 objects. We have seen in previous chapters that energy is one of the fundamental concepts of physics. Q = m × c × δt. Q is the heat energy. to experimentally measure. Calorimeter Experiment Calculations.
From www.chegg.com
Solved /D EXPERIMENT 8 CALORIMETRY 0 Data and Calculations Calorimeter Experiment Calculations We have seen in previous chapters that energy is one of the fundamental concepts of physics. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. calorimetry is used to measure amounts of heat transferred to or from a substance. M is the mass of the. in. Calorimeter Experiment Calculations.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimeter Experiment Calculations To do so, the heat is exchanged with a calibrated. solve problems involving heat transfer. The equation to calculate enthalpy changes from temperature changes is: We use capital cc to represent the heat. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. To apply these \( \delta. Calorimeter Experiment Calculations.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimeter Experiment Calculations We have seen in previous chapters that energy is one of the fundamental concepts of physics. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. calorimetry is used to measure amounts of heat transferred to or from a substance. The equation to. Calorimeter Experiment Calculations.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimeter Experiment Calculations To do so, the heat is exchanged with a calibrated. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. The equation to calculate enthalpy changes from temperature changes is: Q = m × c × δt. Q is the heat energy. We. Calorimeter Experiment Calculations.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimeter Experiment Calculations to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. calorimetry is used to measure amounts of heat transferred to or from a substance. The equation to calculate enthalpy changes from temperature changes is: to make sure you get accurate results you need to calculate the calorimeter constant, which. Calorimeter Experiment Calculations.
From study.com
Calorimetry Definition, Equation & Types Lesson Calorimeter Experiment Calculations It can analyze the heat exchange between up to 3 objects. Q = m × c × δt. To do so, the heat is exchanged with a calibrated. We have seen in previous chapters that energy is one of the fundamental concepts of physics. We use capital cc to represent the heat. to experimentally measure the \( \delta h\). Calorimeter Experiment Calculations.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.2 Describe Simple Calorimetry Experiments for Calorimeter Experiment Calculations experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. We have seen in previous chapters that energy is one of the fundamental concepts of physics. The equation to calculate enthalpy changes from temperature changes is: solve problems involving heat transfer. We use. Calorimeter Experiment Calculations.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Experiment Calculations To do so, the heat is exchanged with a calibrated. To apply these \( \delta h\) values in a. Q = m × c × δt. We use capital cc to represent the heat. M is the mass of the. The equation to calculate enthalpy changes from temperature changes is: It can analyze the heat exchange between up to 3. Calorimeter Experiment Calculations.
From www.nagwa.com
Question Video Identifying the Factor That Does Not Need to Be Held Calorimeter Experiment Calculations to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. calorimetry is used to measure amounts of heat transferred to or from a substance. We have seen in previous chapters that energy is one of the fundamental concepts of physics. The equation to calculate enthalpy changes from temperature. Calorimeter Experiment Calculations.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimeter Experiment Calculations We use capital cc to represent the heat. the calorimetry calculator can help you solve complex calorimetry problems. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. in this experiment you will heat a known mass of a metal to a known temperature and then transfer. Calorimeter Experiment Calculations.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calorimeter Experiment Calculations The equation to calculate enthalpy changes from temperature changes is: Q = m × c × δt. calorimetry is used to measure amounts of heat transferred to or from a substance. Q is the heat energy. We use capital cc to represent the heat. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water. Calorimeter Experiment Calculations.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Experiment Calculations M is the mass of the. to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. Q = m × c × δt. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. in. Calorimeter Experiment Calculations.
From www.bartleby.com
Answered Experiment 14 Data and Calculations… bartleby Calorimeter Experiment Calculations The equation to calculate enthalpy changes from temperature changes is: To do so, the heat is exchanged with a calibrated. solve problems involving heat transfer. Q is the heat energy. We have seen in previous chapters that energy is one of the fundamental concepts of physics. the calorimetry calculator can help you solve complex calorimetry problems. To apply. Calorimeter Experiment Calculations.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimeter Experiment Calculations Q = m × c × δt. calorimetry is used to measure amounts of heat transferred to or from a substance. M is the mass of the. To apply these \( \delta h\) values in a. solve problems involving heat transfer. the calorimetry calculator can help you solve complex calorimetry problems. in this experiment you will. Calorimeter Experiment Calculations.
From www.chegg.com
Solved Experiment 25 Report Sheet Calorimetry Lab Sec Name Calorimeter Experiment Calculations To apply these \( \delta h\) values in a. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. the calorimetry calculator can help you solve complex calorimetry problems. M is the mass of the. calorimetry is used to measure amounts of heat transferred to or from. Calorimeter Experiment Calculations.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeter Experiment Calculations in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. the calorimetry calculator can help you solve complex calorimetry problems. The equation to calculate enthalpy changes from temperature changes is: experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity. Calorimeter Experiment Calculations.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Experiment Calculations to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. calorimetry is used to measure amounts of heat transferred to or from a substance. It can analyze the heat. Calorimeter Experiment Calculations.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Experiment Calculations experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. the calorimetry calculator can help you solve complex calorimetry problems. To apply these \( \delta h\) values in a. in this experiment you will heat a known mass of a metal to. Calorimeter Experiment Calculations.
From users.highland.edu
Calorimetry Calorimeter Experiment Calculations We use capital cc to represent the heat. to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. We have seen in previous chapters that energy is one of the fundamental concepts of physics. To do so, the heat is exchanged with a calibrated. to make sure you get accurate. Calorimeter Experiment Calculations.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts Calorimeter Experiment Calculations Q is the heat energy. solve problems involving heat transfer. M is the mass of the. the calorimetry calculator can help you solve complex calorimetry problems. calorimetry is used to measure amounts of heat transferred to or from a substance. The equation to calculate enthalpy changes from temperature changes is: We have seen in previous chapters that. Calorimeter Experiment Calculations.
From answermagicmatney.z21.web.core.windows.net
Coffee Cup Calorimetry Experiment Calorimeter Experiment Calculations M is the mass of the. Q = m × c × δt. solve problems involving heat transfer. We have seen in previous chapters that energy is one of the fundamental concepts of physics. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Calorimeter Experiment Calculations.
From www.chegg.com
Solved Calculations I. Heat Capacity of the Calorimeter The Calorimeter Experiment Calculations It can analyze the heat exchange between up to 3 objects. M is the mass of the. the calorimetry calculator can help you solve complex calorimetry problems. Q = m × c × δt. calorimetry is used to measure amounts of heat transferred to or from a substance. to make sure you get accurate results you need. Calorimeter Experiment Calculations.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Experiment Calculations The equation to calculate enthalpy changes from temperature changes is: We use capital cc to represent the heat. To apply these \( \delta h\) values in a. to experimentally measure the \( \delta h\) values of two reactions using the technique of constant pressure calorimetry. in this experiment you will heat a known mass of a metal to. Calorimeter Experiment Calculations.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Experiment Calculations in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a. the calorimetry calculator can help you solve complex calorimetry problems. We use capital cc to represent the heat. M is the mass of the. To do so, the heat is exchanged with. Calorimeter Experiment Calculations.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimeter Experiment Calculations the calorimetry calculator can help you solve complex calorimetry problems. calorimetry is used to measure amounts of heat transferred to or from a substance. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. We use capital cc to represent the heat. to experimentally measure the. Calorimeter Experiment Calculations.
From www.youtube.com
Calorimetry calculation YouTube Calorimeter Experiment Calculations We use capital cc to represent the heat. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. M is the mass of the. To do so, the heat is exchanged with a calibrated. We have seen in previous chapters that energy is one of the fundamental concepts of. Calorimeter Experiment Calculations.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Calorimeter Experiment Calculations calorimetry is used to measure amounts of heat transferred to or from a substance. We use capital cc to represent the heat. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. solve problems involving heat transfer. to make sure you. Calorimeter Experiment Calculations.