Medical Device Alerts Definition . Published for joint commission accredited organizations and. Recalls occur when a medical. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. This may include the continuous infusion of medication and. A recall is an action taken to address a problem with a medical device that violates fda law. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. The joint commission medical device alarm safety in hospitals. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a.
from www.cchwyo.org
Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Recalls occur when a medical. A recall is an action taken to address a problem with a medical device that violates fda law. This may include the continuous infusion of medication and. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. The joint commission medical device alarm safety in hospitals. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. Published for joint commission accredited organizations and.
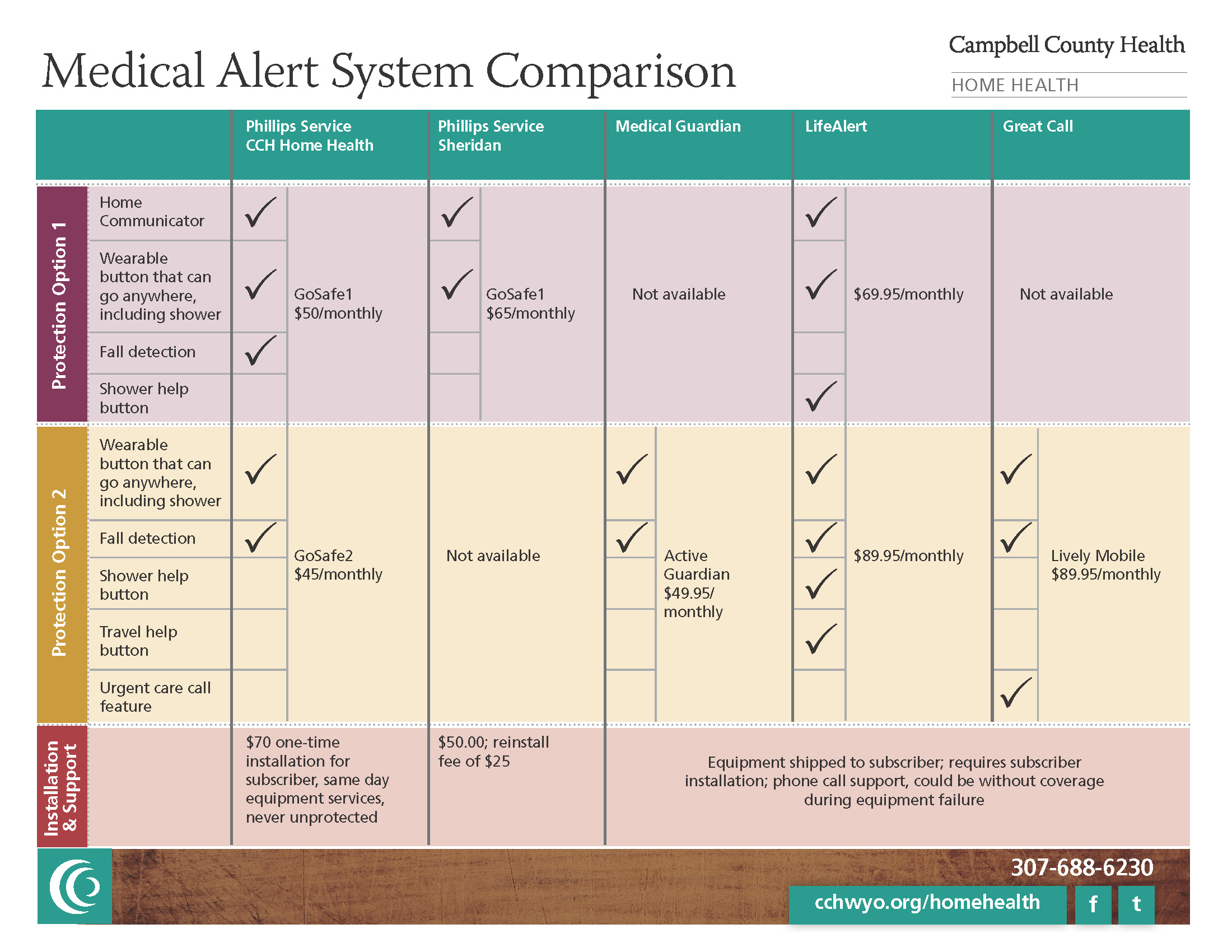
Medical Alert Systems in Gillette, Wyoming
Medical Device Alerts Definition The joint commission medical device alarm safety in hospitals. The joint commission medical device alarm safety in hospitals. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. Published for joint commission accredited organizations and. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. This may include the continuous infusion of medication and. A recall is an action taken to address a problem with a medical device that violates fda law. Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. Recalls occur when a medical.
From studylib.net
Medical Device Alert Medical Device Alerts Definition Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. This may include the continuous infusion of medication and. Recalls occur. Medical Device Alerts Definition.
From www.consumerreports.org
How to Choose a Medical Alert System Consumer Reports Medical Device Alerts Definition The joint commission medical device alarm safety in hospitals. Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. Published for joint commission accredited organizations and. A recall is an action taken to address a problem with a medical device that violates fda law. This may include the continuous infusion of medication and.. Medical Device Alerts Definition.
From www.positivemed.com
Understanding the Basics of a Medical Alert System PositiveMed Medical Device Alerts Definition Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. The joint commission medical device alarm safety in hospitals. This may include the continuous infusion of medication and. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. Medical device recalls typically begin with safety or manufacturing issues. Medical Device Alerts Definition.
From www.medicalalertcomparison.com
Mini Guardian Medical Alert Device Review Medical Device Alerts Definition This may include the continuous infusion of medication and. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. The joint commission medical device alarm safety in hospitals. A recall is an action taken to address a problem with a medical device that violates fda law. Medical device recalls typically begin. Medical Device Alerts Definition.
From aginginplace.org
Best Medical Alert Systems of 2023 Medical Device Alerts Definition Published for joint commission accredited organizations and. A recall is an action taken to address a problem with a medical device that violates fda law. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. Medical device reporting. Medical Device Alerts Definition.
From www.caregiverbliss.com
Medical Alert Systems Glossary Definition Medical Device Alerts Definition Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. Recalls occur when a medical. A recall is an action taken to address a problem with a medical device that violates fda law. Medical alert devices. Medical Device Alerts Definition.
From www.everydayhealth.com
The 5 Best Medical Alert Systems for 2022 Medical Device Alerts Definition Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. This may include the continuous infusion of medication and. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. The joint commission medical device alarm safety in hospitals. Recalls occur when a medical. A recall is. Medical Device Alerts Definition.
From www.pinterest.com
Emergency Alert Device for Elderly Medihill Medical alert system Medical Device Alerts Definition Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. A recall is an action taken to address a problem with a medical device that violates fda law. This may include the continuous infusion of medication and. The joint commission medical device alarm safety in hospitals. Recalls occur when a medical. Medical device reporting (mdr). Medical Device Alerts Definition.
From seniorsmobility.org
Wearable Medical Alert Devices (2022) Types, Top Models + Expert Medical Device Alerts Definition A recall is an action taken to address a problem with a medical device that violates fda law. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Published for joint commission accredited organizations and. This. Medical Device Alerts Definition.
From zaggocare.org
Do You Know About Medical Device Recalls? ZaggoCare Medical Device Alerts Definition This may include the continuous infusion of medication and. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. A recall is an action taken to address a problem with a medical device that violates fda law. Recalls occur when a medical. Medical device recalls typically begin with safety or manufacturing issues identified. Medical Device Alerts Definition.
From medicaldialogues.in
CDSCO issues Medical Device Alert for Abbott MitraClip Device Medical Device Alerts Definition A recall is an action taken to address a problem with a medical device that violates fda law. The joint commission medical device alarm safety in hospitals. Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. Medical. Medical Device Alerts Definition.
From www.youtube.com
Medical Device Alert for Abbott MitraClip Device YouTube Medical Device Alerts Definition Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. A recall is an action taken to address a problem with a medical device that violates fda law. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. Medical alert devices are medical technologies. Medical Device Alerts Definition.
From medicalalertsystemshq.com
LifeLink Prodigy Medical Alert System Review Medical Alert Systems HQ Medical Device Alerts Definition The joint commission medical device alarm safety in hospitals. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. A recall is an action taken to address a problem with a medical device that violates fda law. Published for joint commission accredited organizations and. Medical device recalls typically begin with safety. Medical Device Alerts Definition.
From www.alert-1.com
Inclusive Medical Alert Systems with Fall Detection Alert1 Medical Device Alerts Definition This may include the continuous infusion of medication and. Published for joint commission accredited organizations and. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. A recall is an action taken to address a problem with a medical device that violates fda law. The joint commission medical device alarm safety. Medical Device Alerts Definition.
From present5.com
Medical Device Alert Recall Management Leo de Medical Device Alerts Definition Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Recalls occur when a medical. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. A recall is an action taken to address a problem with a medical device that violates fda law. Published. Medical Device Alerts Definition.
From www.youtube.com
Best Medical Alert System 2022 Medical Alert Devices Personal Medical Device Alerts Definition Recalls occur when a medical. Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. Published for joint commission accredited organizations and. The joint commission medical device alarm safety in hospitals. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. Medical device reporting (mdr) is one of. Medical Device Alerts Definition.
From 24x7mag.com
ECRI, oneSOURCE Collaborating to Improve Medical Device Alerts Medical Device Alerts Definition Published for joint commission accredited organizations and. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. A recall is an action taken to address a problem with a medical device that violates fda law. Recalls occur when a medical. Medical alert devices are medical technologies that tend to the complex physiological monitoring. Medical Device Alerts Definition.
From homeandwellness.com
A smart guideline about Medical Alert Devices Home and Wellness Medical Device Alerts Definition This may include the continuous infusion of medication and. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. A recall is an action taken to address a problem with a medical device that violates fda law. Medical device safety alert an advisory communication by the fda mandating dissemination of information. Medical Device Alerts Definition.
From www.cchwyo.org
Medical Alert Systems in Gillette, Wyoming Medical Device Alerts Definition The joint commission medical device alarm safety in hospitals. A recall is an action taken to address a problem with a medical device that violates fda law. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. This. Medical Device Alerts Definition.
From www.youtube.com
UNBOXING SOS ALLINONE MEDICAL ALERT DEVICE Bay Alarm Medical YouTube Medical Device Alerts Definition Recalls occur when a medical. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. This may include the continuous infusion of medication and. The joint commission medical device alarm safety in hospitals. A recall is. Medical Device Alerts Definition.
From medicalcarealertusa.medium.com
An indepth explanation of the operation of medical alert systems is Medical Device Alerts Definition A recall is an action taken to address a problem with a medical device that violates fda law. The joint commission medical device alarm safety in hospitals. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. Medical. Medical Device Alerts Definition.
From www.shmula.com
Medical Alert Symbol and Visual Management Medical Device Alerts Definition Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. Medical device reporting (mdr) is one of the postmarket surveillance tools. Medical Device Alerts Definition.
From dokumen.tips
(PDF) Medical Device Alert (FINAL) · PDF fileMedical Device Alert Medical Device Alerts Definition Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. Recalls occur when a medical. The joint commission medical device alarm safety in hospitals. This may include the continuous infusion of medication and. Published for joint commission accredited organizations and. Medical alert devices are medical technologies that tend to the complex physiological monitoring. Medical Device Alerts Definition.
From briohouse.com
How Do Medical Alert Systems Work? Brio House Medical Device Alerts Definition Recalls occur when a medical. The joint commission medical device alarm safety in hospitals. This may include the continuous infusion of medication and. A recall is an action taken to address a problem with a medical device that violates fda law. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. Medical device recalls typically. Medical Device Alerts Definition.
From emergencymedicalalarmsystems.wordpress.com
Know About Medical Alerts And Who Should Use It Home medical alert Medical Device Alerts Definition The joint commission medical device alarm safety in hospitals. This may include the continuous infusion of medication and. Published for joint commission accredited organizations and. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance,. Medical Device Alerts Definition.
From www.lifestation.com
LifeStation Medical Alert AFSCME Advantage Medical Device Alerts Definition This may include the continuous infusion of medication and. Recalls occur when a medical. The joint commission medical device alarm safety in hospitals. Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. Published for joint. Medical Device Alerts Definition.
From ashtons.com
Managing medicines safety alerts new MHRA Central Alerting System Medical Device Alerts Definition Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. A recall is an action taken to address a problem with a medical device that violates fda law. Recalls occur when a medical. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. Medical device recalls typically begin. Medical Device Alerts Definition.
From www.reviews.com
Best Medical Alert Systems of 2017 Medical Device Alerts Definition Recalls occur when a medical. The joint commission medical device alarm safety in hospitals. Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. A recall is an action taken to address a problem with a medical device that violates fda law. This may include the continuous infusion of medication and. Medical alert. Medical Device Alerts Definition.
From www.everydayhealth.com
The 5 Best Medical Alert Systems for 2022 Medical Device Alerts Definition Recalls occur when a medical. The joint commission medical device alarm safety in hospitals. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. Published for joint commission accredited organizations and. Medical alert devices. Medical Device Alerts Definition.
From choicemutual.com
Complete Senior Guide To Medical Alert Systems In 2021 Medical Device Alerts Definition Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. Published for joint commission accredited organizations and. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. This may include the continuous infusion of medication and. The joint commission medical device alarm safety in hospitals. Medical. Medical Device Alerts Definition.
From www.checkbook.org
Medical Alert Devices Washington Consumers' Checkbook Medical Device Alerts Definition Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. This may include the continuous infusion of medication and. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect.. Medical Device Alerts Definition.
From www.yumpu.com
Medical Device ALERT Department of Health Medical Device Alerts Definition Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. Medical device recalls typically begin with safety or manufacturing issues identified by the device maker, physicians or. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. Medical device reporting (mdr) is one of the postmarket surveillance tools. Medical Device Alerts Definition.
From www.scribd.com
Medical Device Alert PDF National Health Service Health Care Medical Device Alerts Definition The joint commission medical device alarm safety in hospitals. This may include the continuous infusion of medication and. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. A recall is an action taken to address a problem with a medical device that violates fda law. Medical device recalls typically begin with safety or manufacturing. Medical Device Alerts Definition.
From www.reviews.com
The Best Medical Alert Systems of 2018 Medical Device Alerts Definition Recalls occur when a medical. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance, detect. Medical device safety alert an advisory communication by the fda mandating dissemination of information indicating that a. Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. The joint commission medical. Medical Device Alerts Definition.
From www.forbes.com
Best Medical Alert Systems With Fall Detection (2024) Forbes Health Medical Device Alerts Definition Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. This may include the continuous infusion of medication and. A recall is an action taken to address a problem with a medical device that violates fda law. Medical device reporting (mdr) is one of the postmarket surveillance tools the fda uses to monitor device performance,. Medical Device Alerts Definition.