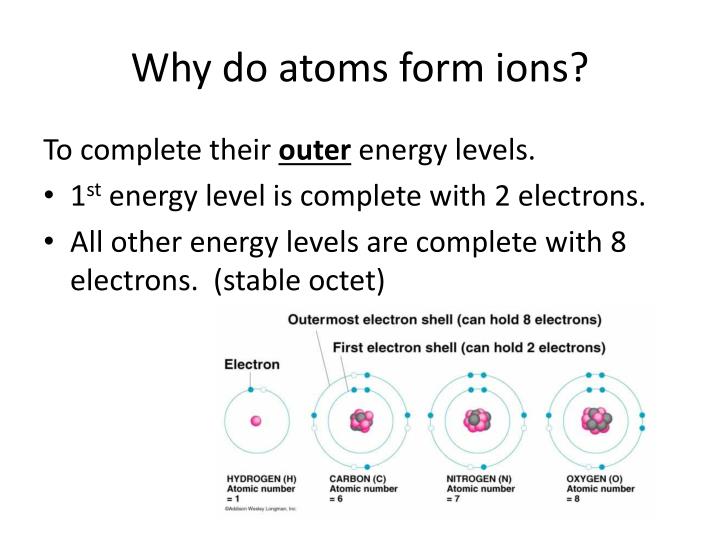
Why Do Atoms Have A Positive Charge . Protons have a positive electric charge and neutrons have no charge, so the nucleus is. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. When they do, they form charged particles called ions : Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. If an atom loses one or more electrons, it becomes a positively charged ion Atoms can lose or gain electrons. As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry no charge;. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. More than 99.94% of an atom's mass is in the nucleus.
from www.slideserve.com
Protons have a positive electric charge and neutrons have no charge, so the nucleus is. When they do, they form charged particles called ions : As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry no charge;. More than 99.94% of an atom's mass is in the nucleus. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Atoms can lose or gain electrons. If an atom loses one or more electrons, it becomes a positively charged ion
PPT Ion Formation PowerPoint Presentation ID2508414
Why Do Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electric charge and neutrons have no charge, so the nucleus is. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. When they do, they form charged particles called ions : If an atom loses one or more electrons, it becomes a positively charged ion As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry no charge;. Atoms can lose or gain electrons. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. More than 99.94% of an atom's mass is in the nucleus.
From electricallive.com
Atomic Structure And Electric Charge Electrical engineering interview Why Do Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Atoms can lose or gain electrons. If an atom loses one or more electrons, it becomes. Why Do Atoms Have A Positive Charge.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation ID2508414 Why Do Atoms Have A Positive Charge Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry. Why Do Atoms Have A Positive Charge.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation Why Do Atoms Have A Positive Charge Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. When they do, they form charged particles called ions : Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electric charge and. Why Do Atoms Have A Positive Charge.
From www.slideserve.com
PPT What part of an atom is the arrow pointing to? PowerPoint Why Do Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. When they do, they form charged particles called ions : Protons have a positive electric charge. Why Do Atoms Have A Positive Charge.
From www.masterorganicchemistry.com
7 Factors That Stabilize Positive Charge in Organic Chemistry Master Why Do Atoms Have A Positive Charge Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Atoms can lose or gain electrons. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Protons have a positive electrical charge of one \(\left( +1 \right)\). Why Do Atoms Have A Positive Charge.
From www.slideserve.com
PPT Protons are positive (+) charged particles found in the nucleus Why Do Atoms Have A Positive Charge When they do, they form charged particles called ions : Atoms can lose or gain electrons. If an atom loses one or more electrons, it becomes a positively charged ion Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Protons have a positive electrical charge of one. Why Do Atoms Have A Positive Charge.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Why Do Atoms Have A Positive Charge As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry no charge;. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electric charge and neutrons have no charge, so the nucleus is. Electrons contribute greatly to the. Why Do Atoms Have A Positive Charge.
From solbergsphyscience.wikispaces.com
solbergsphyscience The Structure of the Atom Why Do Atoms Have A Positive Charge As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry no charge;. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu}. Why Do Atoms Have A Positive Charge.
From peyton-has-tapia.blogspot.com
An Atom With a Net Positive Charge Must Have More PeytonhasTapia Why Do Atoms Have A Positive Charge Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Atoms can lose or gain electrons. Protons have a positive electric charge and neutrons have no charge, so the nucleus is. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass. Why Do Atoms Have A Positive Charge.
From www.masterorganicchemistry.com
7 Factors That Stabilize Positive Charge in Organic Chemistry Master Why Do Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry no. Why Do Atoms Have A Positive Charge.
From byjus.com
In water, oxygen has a slight negative charge and hydrogen has a slight Why Do Atoms Have A Positive Charge Protons have a positive electric charge and neutrons have no charge, so the nucleus is. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry no charge;. More than 99.94% of an. Why Do Atoms Have A Positive Charge.
From circuitpennhockey01yh.z13.web.core.windows.net
Atom With Electrical Charge Why Do Atoms Have A Positive Charge If an atom loses one or more electrons, it becomes a positively charged ion Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Protons have a. Why Do Atoms Have A Positive Charge.
From www.chemistrystudent.com
Atomic Structure (ALevel) ChemistryStudent Why Do Atoms Have A Positive Charge More than 99.94% of an atom's mass is in the nucleus. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electric charge and neutrons have no charge, so the nucleus is. Protons have a positive electrical charge of one \(\left( +1 \right)\) and. Why Do Atoms Have A Positive Charge.
From www.youtube.com
how an atom gets positively charged YouTube Why Do Atoms Have A Positive Charge More than 99.94% of an atom's mass is in the nucleus. Atoms can lose or gain electrons. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive.. Why Do Atoms Have A Positive Charge.
From www.sliderbase.com
Formula of Ionic Compounds Why Do Atoms Have A Positive Charge When they do, they form charged particles called ions : Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. As their names suggest, protons have. Why Do Atoms Have A Positive Charge.
From www.slideserve.com
PPT Chapter 21 Electric Charge and Electric Fields PowerPoint Why Do Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. When they do, they form charged particles called ions : Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Protons have a positive electrical charge. Why Do Atoms Have A Positive Charge.
From www.sliderbase.com
Atoms and the Periodic table Presentation Chemistry Why Do Atoms Have A Positive Charge As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry no charge;. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Why Do Atoms Have A Positive Charge.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Why Do Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry no charge;. When they do, they form charged particles called ions : Protons have a positive electric charge and neutrons have no. Why Do Atoms Have A Positive Charge.
From edurev.in
Structure of Atom Class 9 Notes EduRev Why Do Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Atoms can lose or gain electrons. Protons have a positive electric charge and neutrons have no charge, so the nucleus is. If an atom loses one or more electrons, it becomes a positively charged ion Protons have a. Why Do Atoms Have A Positive Charge.
From www.expii.com
Hydrogen Bonds — Overview & Examples Expii Why Do Atoms Have A Positive Charge More than 99.94% of an atom's mass is in the nucleus. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Protons have a positive electrical charge. Why Do Atoms Have A Positive Charge.
From slideplayer.com
Chapter 6 Section ppt download Why Do Atoms Have A Positive Charge More than 99.94% of an atom's mass is in the nucleus. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. When they do, they form charged particles called ions : Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic. Why Do Atoms Have A Positive Charge.
From www.slideserve.com
PPT Lecture 1 Charge and Coulomb’s Law PowerPoint Presentation, free Why Do Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. When they do, they form charged particles called ions : Protons have a positive electric charge and neutrons have no charge, so the nucleus is. Atoms can lose or gain electrons. If an atom loses one or more. Why Do Atoms Have A Positive Charge.
From design.udlvirtual.edu.pe
What Does It Mean When An Ion Has A Positive Charge Design Talk Why Do Atoms Have A Positive Charge More than 99.94% of an atom's mass is in the nucleus. When they do, they form charged particles called ions : Protons have a positive electric charge and neutrons have no charge, so the nucleus is. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Protons have a. Why Do Atoms Have A Positive Charge.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation ID2508414 Why Do Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. If an atom loses one or more electrons, it becomes a positively charged ion Atoms can lose or gain electrons. As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry no charge;.. Why Do Atoms Have A Positive Charge.
From slideplayer.com
Chapter 6 Section ppt download Why Do Atoms Have A Positive Charge Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. More than 99.94% of an atom's mass is in the nucleus. If an atom loses one or. Why Do Atoms Have A Positive Charge.
From slideplayer.com
Why do atoms combine? 1 Atomic Structures ppt download Why Do Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Atoms can lose or gain electrons. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. As their names suggest, protons have a positive electrical charge,. Why Do Atoms Have A Positive Charge.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Why Do Atoms Have A Positive Charge If an atom loses one or more electrons, it becomes a positively charged ion Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry no charge;. Atoms can lose or gain electrons.. Why Do Atoms Have A Positive Charge.
From www.slideserve.com
PPT Protons are positive (+) charged particles found in the nucleus Why Do Atoms Have A Positive Charge If an atom loses one or more electrons, it becomes a positively charged ion As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry no charge;. Protons have a positive electric charge and neutrons have no charge, so the nucleus is. Atoms can lose or gain electrons. When they do, they form charged particles. Why Do Atoms Have A Positive Charge.
From www.nagwa.com
Question Video Recalling the Name of the Positively Charged Particles Why Do Atoms Have A Positive Charge Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. If an atom loses one or more electrons, it becomes a positively charged ion More than 99.94%. Why Do Atoms Have A Positive Charge.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Why Do Atoms Have A Positive Charge When they do, they form charged particles called ions : Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Atoms that lose electrons acquire a. Why Do Atoms Have A Positive Charge.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation ID2508414 Why Do Atoms Have A Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. When they do, they form charged particles called ions : If an atom loses one or more. Why Do Atoms Have A Positive Charge.
From telgurus.co.uk
What is the overall charge of the nucleus of an atom and why? Why Do Atoms Have A Positive Charge If an atom loses one or more electrons, it becomes a positively charged ion Protons have a positive electric charge and neutrons have no charge, so the nucleus is. More than 99.94% of an atom's mass is in the nucleus. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left(. Why Do Atoms Have A Positive Charge.
From worksheetfulldisbosom.z22.web.core.windows.net
Parts Of An Atom Why Do Atoms Have A Positive Charge When they do, they form charged particles called ions : If an atom loses one or more electrons, it becomes a positively charged ion Protons have a positive electric charge and neutrons have no charge, so the nucleus is. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive.. Why Do Atoms Have A Positive Charge.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Why Do Atoms Have A Positive Charge As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry no charge;. Atoms can lose or gain electrons. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Atoms that lose electrons acquire a positive charge because they are left with fewer. Why Do Atoms Have A Positive Charge.
From periodictable.me
What are the Difference Between Charge and Electron? Dynamic Periodic Why Do Atoms Have A Positive Charge Protons have a positive electric charge and neutrons have no charge, so the nucleus is. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry no charge;. Protons have a positive electrical. Why Do Atoms Have A Positive Charge.