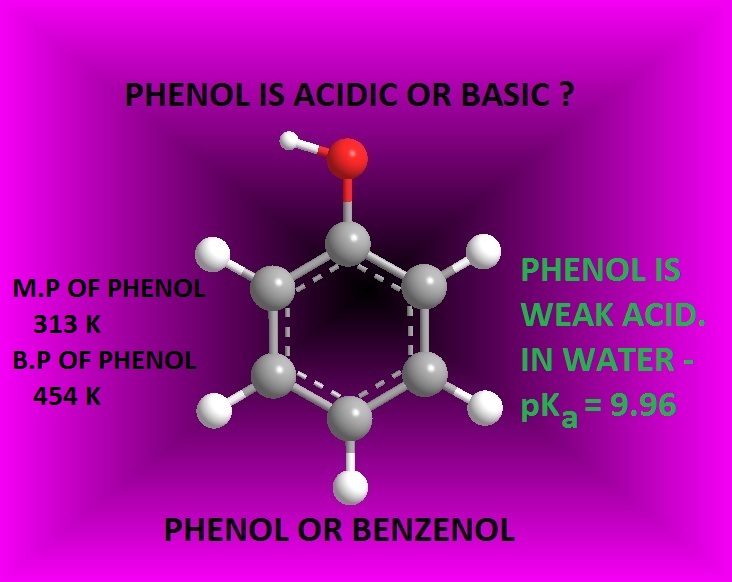
Why Phenol Is Acidic In Nature . Happening such reactions of phenols with metals indicates it is acidic in nature. The increased acidity of phenol is caused by the. Learn how phenol reacts with bases, carbonates and sodium. Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (naoh), to form the phenoxide ion. The acidity of phenols depends on the resonance,. This page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Phenols also react with aqueous sodium hydroxide to produce.
from chemizi.blogspot.com
The acidity of phenols depends on the resonance,. Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (naoh), to form the phenoxide ion. This page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. Happening such reactions of phenols with metals indicates it is acidic in nature. Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. The increased acidity of phenol is caused by the. Phenols also react with aqueous sodium hydroxide to produce. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Learn how phenol reacts with bases, carbonates and sodium. Delocalization of the negative charge over the ortho and para positions of the aromatic ring.
Phenol is acidic or basic and Which is stronger acid phenol or cresol
Why Phenol Is Acidic In Nature The increased acidity of phenol is caused by the. Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (naoh), to form the phenoxide ion. The acidity of phenols depends on the resonance,. This page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Phenols also react with aqueous sodium hydroxide to produce. The increased acidity of phenol is caused by the. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. Learn how phenol reacts with bases, carbonates and sodium. Happening such reactions of phenols with metals indicates it is acidic in nature.
From www.toppr.com
Explain acidic nature of phenol with the help of reactions. Why Phenol Is Acidic In Nature Happening such reactions of phenols with metals indicates it is acidic in nature. The increased acidity of phenol is caused by the. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Learn how phenol reacts with bases, carbonates and sodium. Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide. Why Phenol Is Acidic In Nature.
From www.youtube.com
Alcohol, Phenol & Ether 4 Physical Properties Chemical Why Phenol Is Acidic In Nature Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenols also react with aqueous sodium hydroxide to produce. Happening such reactions of phenols with metals indicates it is acidic in nature. This page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases. Why Phenol Is Acidic In Nature.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID4524523 Why Phenol Is Acidic In Nature Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (naoh), to form the phenoxide ion. Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. The increased acidity of phenol is caused by the. In. Why Phenol Is Acidic In Nature.
From chemizi.blogspot.com
Phenol is acidic in naturephenol to salicylic acid and benzene change Why Phenol Is Acidic In Nature This page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Happening such reactions of phenols with metals indicates it is acidic in nature. Learn how phenol reacts with bases,. Why Phenol Is Acidic In Nature.
From chemizi.blogspot.com
Phenol is acidic in naturephenol to salicylic acid and benzene change Why Phenol Is Acidic In Nature The acidity of phenols depends on the resonance,. Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (naoh), to form the phenoxide ion. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenols also react. Why Phenol Is Acidic In Nature.
From www.youtube.com
why carboxylic acid more acidic than phenols/Acidi nature of carboxylic Why Phenol Is Acidic In Nature Learn how phenol reacts with bases, carbonates and sodium. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Phenols also react with aqueous sodium hydroxide to produce. Happening such reactions of phenols with metals indicates it is acidic in nature. Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (naoh),. Why Phenol Is Acidic In Nature.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Why Phenol Is Acidic In Nature In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. The increased acidity of phenol is caused by the. Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (naoh), to form the phenoxide ion. Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. Delocalization of. Why Phenol Is Acidic In Nature.
From www.youtube.com
Why Phenol is Acidic in Nature? YouTube Why Phenol Is Acidic In Nature Happening such reactions of phenols with metals indicates it is acidic in nature. This page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. The increased acidity of phenol is caused by the. Phenols also react with aqueous sodium hydroxide to produce. In aqueous. Why Phenol Is Acidic In Nature.
From chemizi.blogspot.com
Phenol is acidic or basic and Which is stronger acid phenol or cresol Why Phenol Is Acidic In Nature Happening such reactions of phenols with metals indicates it is acidic in nature. Learn how phenol reacts with bases, carbonates and sodium. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. This page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and. Why Phenol Is Acidic In Nature.
From www.youtube.com
🔥Phenol Bsc 2nd year🔥 Why Phenol is Acidic in Nature 🔥 Important Why Phenol Is Acidic In Nature The increased acidity of phenol is caused by the. Phenols also react with aqueous sodium hydroxide to produce. Happening such reactions of phenols with metals indicates it is acidic in nature. Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (naoh), to form the phenoxide ion. This page explains why phenol is a weak acid. Why Phenol Is Acidic In Nature.
From www.toppr.com
Explain why phenol is more acidic than ethanol? Why Phenol Is Acidic In Nature The acidity of phenols depends on the resonance,. Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (naoh), to form the phenoxide ion. Happening such reactions of phenols with metals indicates it is acidic in nature. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Delocalization of the negative charge. Why Phenol Is Acidic In Nature.
From www.youtube.com
Why phenol is acidic in nature? organicchemistry phenols shorts Why Phenol Is Acidic In Nature Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (naoh), to form the phenoxide ion. This page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. The acidity of phenols depends on the resonance,. The increased acidity of phenol. Why Phenol Is Acidic In Nature.
From byjus.com
Phenols are more acidic than alcohol. Explain why? Why Phenol Is Acidic In Nature Phenols also react with aqueous sodium hydroxide to produce. This page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. The increased acidity of phenol is caused by the.. Why Phenol Is Acidic In Nature.
From chemizi.blogspot.com
Phenol is acidic in naturephenol to salicylic acid and benzene change Why Phenol Is Acidic In Nature The increased acidity of phenol is caused by the. Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (naoh), to form the phenoxide ion. Phenols also react with aqueous sodium hydroxide to produce. Happening such reactions of phenols with metals indicates it is acidic in nature. In aqueous solutions, phenols are weakly acidic and lower. Why Phenol Is Acidic In Nature.
From www.youtube.com
15) Acidic nature of phenol Why Phenol is more acidic than Alcohol Why Phenol Is Acidic In Nature The acidity of phenols depends on the resonance,. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenols also react with aqueous sodium hydroxide to produce. Learn how phenol reacts with bases, carbonates and sodium. This page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack. Why Phenol Is Acidic In Nature.
From www.youtube.com
Acidic nature of phenol most important topic lecture5 YouTube Why Phenol Is Acidic In Nature Phenols also react with aqueous sodium hydroxide to produce. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Learn how phenol reacts with bases, carbonates and sodium. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. The increased acidity of phenol is caused by the. The acidity of phenols. Why Phenol Is Acidic In Nature.
From sciencebyjus.blogspot.com
Acidic Nature of Phenols? Why phenols are more acidic than alcohol Why Phenol Is Acidic In Nature Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. Happening such reactions of phenols with metals indicates it is acidic in nature. The acidity of phenols depends on the resonance,. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenol is acidic enough to be deprotonated by weaker. Why Phenol Is Acidic In Nature.
From byjus.com
What is the order of acidity of alcohols and phenols? Explain both in Why Phenol Is Acidic In Nature This page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. The acidity of phenols depends on the resonance,. Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. Happening such reactions of phenols with metals indicates it is. Why Phenol Is Acidic In Nature.
From chemizi.blogspot.com
Phenol is acidic in naturephenol to salicylic acid and benzene change Why Phenol Is Acidic In Nature Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. Happening such reactions of phenols with metals indicates it is acidic in nature. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Phenols also react with aqueous sodium hydroxide to produce. Delocalization of the negative charge over the ortho and. Why Phenol Is Acidic In Nature.
From www.youtube.com
Acidic Nature of Phenol, why phenol is less acidic than carboxylic Acid Why Phenol Is Acidic In Nature In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Happening such reactions of phenols with metals indicates it is acidic in nature. Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (naoh), to form the phenoxide ion. This page explains why phenol is a weak acid and looks at its. Why Phenol Is Acidic In Nature.
From chemisfast.blogspot.com
Why phenol or benzenol is acidic and Why carboxylic acid is more acidic Why Phenol Is Acidic In Nature The increased acidity of phenol is caused by the. Learn how phenol reacts with bases, carbonates and sodium. Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. Phenols also react with aqueous sodium hydroxide to produce. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Happening such reactions of. Why Phenol Is Acidic In Nature.
From byjus.com
Compare acidic nature A)Ortho amino phenol and meta amino phenol.does h Why Phenol Is Acidic In Nature Phenols also react with aqueous sodium hydroxide to produce. Learn how phenol reacts with bases, carbonates and sodium. Happening such reactions of phenols with metals indicates it is acidic in nature. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Delocalization of the negative charge over the ortho and para positions of the aromatic ring.. Why Phenol Is Acidic In Nature.
From byjus.com
Phenols are more acidic than alcohol. Explain why? Why Phenol Is Acidic In Nature In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. The acidity of phenols depends on the resonance,. The increased acidity of phenol is caused by the. Phenols also react with aqueous sodium hydroxide to produce. Happening such reactions of phenols with metals indicates it is acidic in nature. This page explains why phenol is a. Why Phenol Is Acidic In Nature.
From byjus.com
What is the order of acidity of alcohols and phenols? Explain both in Why Phenol Is Acidic In Nature Delocalization of the negative charge over the ortho and para positions of the aromatic ring. The increased acidity of phenol is caused by the. Learn how phenol reacts with bases, carbonates and sodium. This page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal.. Why Phenol Is Acidic In Nature.
From www.doubtnut.com
(a) Why acidic nature of alcohol and phenol increase with electr Why Phenol Is Acidic In Nature The acidity of phenols depends on the resonance,. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Happening such reactions of phenols with metals indicates it is acidic in nature. The increased acidity of phenol is caused by the. Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (naoh),. Why Phenol Is Acidic In Nature.
From www.youtube.com
Give two reactions that show the acidic nature of phenol. Compare Why Phenol Is Acidic In Nature Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (naoh), to form the phenoxide ion. Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. Happening such reactions of phenols with metals indicates it is acidic in nature. Delocalization of the negative charge over the ortho and para positions of. Why Phenol Is Acidic In Nature.
From www.youtube.com
Alcohol, Phenol & Ether 8 Preparation of Phenol Acidic nature of Why Phenol Is Acidic In Nature Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. This page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. Phenol is acidic enough to be deprotonated by weaker bases, such as sodium hydroxide (naoh), to form the. Why Phenol Is Acidic In Nature.
From www.slideserve.com
PPT PHENOLS PowerPoint Presentation, free download ID9445745 Why Phenol Is Acidic In Nature Happening such reactions of phenols with metals indicates it is acidic in nature. Phenols also react with aqueous sodium hydroxide to produce. Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. In aqueous solutions, phenols are weakly acidic and. Why Phenol Is Acidic In Nature.
From chemizi.blogspot.com
Phenol is acidic in naturephenol to salicylic acid and benzene change Why Phenol Is Acidic In Nature Happening such reactions of phenols with metals indicates it is acidic in nature. The acidity of phenols depends on the resonance,. Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. This page explains why phenol is a weak acid. Why Phenol Is Acidic In Nature.
From www.slideserve.com
PPT Organic Chemistry II Alcohols, Phenols, Thiols , Ethers, and Why Phenol Is Acidic In Nature The increased acidity of phenol is caused by the. Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. Learn how phenol reacts with bases, carbonates and sodium. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. The acidity of phenols depends on the resonance,. Phenol is acidic enough to. Why Phenol Is Acidic In Nature.
From www.doubtnut.com
Phenols are more acidic than aliphatic alcohols acidity of phen Why Phenol Is Acidic In Nature Happening such reactions of phenols with metals indicates it is acidic in nature. This page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. Phenols also react with aqueous sodium hydroxide to produce. Delocalization of the negative charge over the ortho and para positions. Why Phenol Is Acidic In Nature.
From www.youtube.com
Acidic nature of Alcohols and phenols why phenols are more acidic Why Phenol Is Acidic In Nature The increased acidity of phenol is caused by the. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Happening such reactions of phenols with metals indicates it is acidic in nature. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenols are acidic in nature because they can lose. Why Phenol Is Acidic In Nature.
From byjus.com
Why phenol is a weak acid? Why Phenol Is Acidic In Nature Phenols also react with aqueous sodium hydroxide to produce. Learn how phenol reacts with bases, carbonates and sodium. The acidity of phenols depends on the resonance,. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. The increased acidity of phenol is caused by the. This page explains why phenol is a weak acid and. Why Phenol Is Acidic In Nature.
From www.youtube.com
14) Acidic nature of Alcohols why Water is more acidic than Alcohols Why Phenol Is Acidic In Nature Happening such reactions of phenols with metals indicates it is acidic in nature. Learn how phenol reacts with bases, carbonates and sodium. The acidity of phenols depends on the resonance,. Phenols also react with aqueous sodium hydroxide to produce. Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. The increased acidity of phenol is. Why Phenol Is Acidic In Nature.
From www.youtube.com
ACIDIC NATURE OF PHENOL ACIDITY OF PHENOLS PHENOL ACIDITY ORDER Why Phenol Is Acidic In Nature In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Phenols are acidic in nature because they can lose hydrogen ions to form phenoxide ions. This page explains why phenol is a weak acid and looks at its reactions (or in some cases, lack of reaction) with bases and with sodium metal. Delocalization of the negative. Why Phenol Is Acidic In Nature.