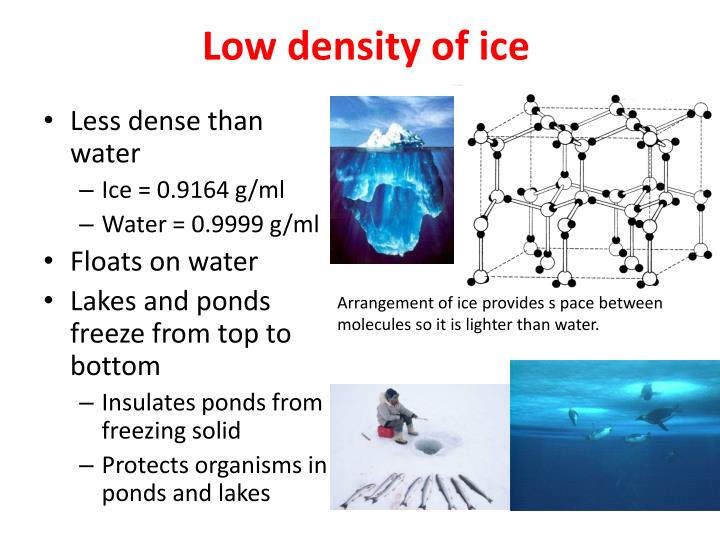
Explain Why Ice Is Less Dense Than Water . So, even though water is more dense than ice, ice still. The density of ice is less than water. It is less dense as a solid than as a liquid, because its particles move apart slightly on freezing. Ponds or lakes begin to freeze at the surface, closer to the cold air. The density of ice, on the other hand, is about 0.9 g/cm^3. All of this physics comes. The density of water is about 1 gram per cubic centimeter (g/cm^3). Ice is less dense than liquid water and so it floats. Structure of ice is a regular open framework of water molecules arranged like honeycomb. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. If you put pressure on regular ice, and give it time to rearrange, the molecules will move into a. Water is different from most substances. Yes, some ice is denser than water. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. Hydrogen bonds hold water molecules in place in solid phase.
from www.slideserve.com
This is due to the water expanding as it is frozen because of the hydrogen forming an open type. The density of ice is less than water. Water is different from most substances. The density of ice, on the other hand, is about 0.9 g/cm^3. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. Ponds or lakes begin to freeze at the surface, closer to the cold air. The density of water is about 1 gram per cubic centimeter (g/cm^3). Ice is less dense than liquid water and so it floats. A layer of ice forms, but does not sink as it would if water did not have this unique structure dictated by its shape, polarity, and hydrogen bonding. If you put pressure on regular ice, and give it time to rearrange, the molecules will move into a.
PPT Understanding Water PowerPoint Presentation ID2739092
Explain Why Ice Is Less Dense Than Water If you put pressure on regular ice, and give it time to rearrange, the molecules will move into a. A layer of ice forms, but does not sink as it would if water did not have this unique structure dictated by its shape, polarity, and hydrogen bonding. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. Ponds or lakes begin to freeze at the surface, closer to the cold air. If you put pressure on regular ice, and give it time to rearrange, the molecules will move into a. The density of ice is less than water. Ice is less dense than liquid water and so it floats. So, even though water is more dense than ice, ice still. All of this physics comes. The density of water is about 1 gram per cubic centimeter (g/cm^3). So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. The density of ice, on the other hand, is about 0.9 g/cm^3. Yes, some ice is denser than water. Hydrogen bonds hold water molecules in place in solid phase. It is less dense as a solid than as a liquid, because its particles move apart slightly on freezing. Water is different from most substances.
From www.slideshare.net
Water Review Explain Why Ice Is Less Dense Than Water Ice is less dense than liquid water and so it floats. All of this physics comes. It is less dense as a solid than as a liquid, because its particles move apart slightly on freezing. Ponds or lakes begin to freeze at the surface, closer to the cold air. A layer of ice forms, but does not sink as it. Explain Why Ice Is Less Dense Than Water.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Explain Why Ice Is Less Dense Than Water Ponds or lakes begin to freeze at the surface, closer to the cold air. Yes, some ice is denser than water. If you put pressure on regular ice, and give it time to rearrange, the molecules will move into a. The density of ice is less than water. All of this physics comes. So, even though water is more dense. Explain Why Ice Is Less Dense Than Water.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, Explain Why Ice Is Less Dense Than Water It is less dense as a solid than as a liquid, because its particles move apart slightly on freezing. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. All of this physics comes. So, the buoyant force will balance out the force of gravity if the density of. Explain Why Ice Is Less Dense Than Water.
From johnnyholland.org
Why is Ice Less Dense Than Water? Johnny Holland Explain Why Ice Is Less Dense Than Water Ice is less dense than liquid water and so it floats. Hydrogen bonds hold water molecules in place in solid phase. Ponds or lakes begin to freeze at the surface, closer to the cold air. Water is different from most substances. Yes, some ice is denser than water. So, the buoyant force will balance out the force of gravity if. Explain Why Ice Is Less Dense Than Water.
From www.slideserve.com
PPT Water Chemistry & Properties of Water PowerPoint Presentation Explain Why Ice Is Less Dense Than Water Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. All of this physics comes. The density of ice, on the other hand, is about 0.9 g/cm^3. Ice is less dense than liquid water and so it floats. This is due to the water expanding as it is frozen. Explain Why Ice Is Less Dense Than Water.
From www.youtube.com
DENSITY OF ICE YouTube Explain Why Ice Is Less Dense Than Water This is due to the water expanding as it is frozen because of the hydrogen forming an open type. A layer of ice forms, but does not sink as it would if water did not have this unique structure dictated by its shape, polarity, and hydrogen bonding. The density of ice is less than water. Ponds or lakes begin to. Explain Why Ice Is Less Dense Than Water.
From byjus.com
Why is density of ice less than water? Explain Why Ice Is Less Dense Than Water Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. The density of water is about 1 gram per cubic centimeter (g/cm^3). Water is different from most substances. A layer of ice forms, but does not sink as it would if water did not have this unique structure dictated. Explain Why Ice Is Less Dense Than Water.
From www.thoughtco.com
Why Is Water More Dense Than Ice? Explain Why Ice Is Less Dense Than Water If you put pressure on regular ice, and give it time to rearrange, the molecules will move into a. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. The density of ice, on the other hand, is about 0.9 g/cm^3. Ice is less denser than. Explain Why Ice Is Less Dense Than Water.
From www.slideshare.net
Unit 1 Notes Explain Why Ice Is Less Dense Than Water If you put pressure on regular ice, and give it time to rearrange, the molecules will move into a. Hydrogen bonds hold water molecules in place in solid phase. Structure of ice is a regular open framework of water molecules arranged like honeycomb. So, even though water is more dense than ice, ice still. Water is different from most substances.. Explain Why Ice Is Less Dense Than Water.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Explain Why Ice Is Less Dense Than Water It is less dense as a solid than as a liquid, because its particles move apart slightly on freezing. Structure of ice is a regular open framework of water molecules arranged like honeycomb. Ice is less dense than liquid water and so it floats. Ponds or lakes begin to freeze at the surface, closer to the cold air. All of. Explain Why Ice Is Less Dense Than Water.
From slideplayer.com
Matter is made of elements ppt download Explain Why Ice Is Less Dense Than Water So, even though water is more dense than ice, ice still. The density of ice is less than water. Ice is less dense than liquid water and so it floats. It is less dense as a solid than as a liquid, because its particles move apart slightly on freezing. Ponds or lakes begin to freeze at the surface, closer to. Explain Why Ice Is Less Dense Than Water.
From www.slideserve.com
PPT Chapter 11 Water and Aqueous Systems PowerPoint Presentation Explain Why Ice Is Less Dense Than Water Structure of ice is a regular open framework of water molecules arranged like honeycomb. So, even though water is more dense than ice, ice still. The density of water is about 1 gram per cubic centimeter (g/cm^3). All of this physics comes. The density of ice is less than water. A layer of ice forms, but does not sink as. Explain Why Ice Is Less Dense Than Water.
From www.slideserve.com
PPT The Science of Water PowerPoint Presentation, free download ID Explain Why Ice Is Less Dense Than Water Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. A layer of ice forms, but does not sink as it would if water did not have this unique structure dictated by its shape, polarity, and hydrogen bonding. Hydrogen bonds hold water molecules in place in solid phase. So,. Explain Why Ice Is Less Dense Than Water.
From www.slideserve.com
PPT The Science of Water PowerPoint Presentation, free download ID Explain Why Ice Is Less Dense Than Water All of this physics comes. Ponds or lakes begin to freeze at the surface, closer to the cold air. The density of ice, on the other hand, is about 0.9 g/cm^3. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. The density of water is about 1 gram per cubic. Explain Why Ice Is Less Dense Than Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Explain Why Ice Is Less Dense Than Water It is less dense as a solid than as a liquid, because its particles move apart slightly on freezing. The density of ice is less than water. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. So, the buoyant force will balance out the force of gravity if the density. Explain Why Ice Is Less Dense Than Water.
From thefactbase.com
Water expands when it freezes ice has a lesser density than water so an Explain Why Ice Is Less Dense Than Water All of this physics comes. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. The density of ice is less than water. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. The density of water is about 1. Explain Why Ice Is Less Dense Than Water.
From byjus.com
Explain density of ice is less than water with molecular structure Explain Why Ice Is Less Dense Than Water It is less dense as a solid than as a liquid, because its particles move apart slightly on freezing. Structure of ice is a regular open framework of water molecules arranged like honeycomb. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. So, even though. Explain Why Ice Is Less Dense Than Water.
From www.slideserve.com
PPT Hydrogen bonding PowerPoint Presentation ID2452680 Explain Why Ice Is Less Dense Than Water Ponds or lakes begin to freeze at the surface, closer to the cold air. The density of ice is less than water. Structure of ice is a regular open framework of water molecules arranged like honeycomb. Ice is less dense than liquid water and so it floats. Hydrogen bonds hold water molecules in place in solid phase. All of this. Explain Why Ice Is Less Dense Than Water.
From www.chegg.com
Solved Which statement helps to explain why ice is less Explain Why Ice Is Less Dense Than Water A layer of ice forms, but does not sink as it would if water did not have this unique structure dictated by its shape, polarity, and hydrogen bonding. So, even though water is more dense than ice, ice still. All of this physics comes. The density of ice is less than water. So, the buoyant force will balance out the. Explain Why Ice Is Less Dense Than Water.
From www.slideserve.com
PPT The Science of Water PowerPoint Presentation, free download ID Explain Why Ice Is Less Dense Than Water The density of ice, on the other hand, is about 0.9 g/cm^3. So, even though water is more dense than ice, ice still. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. The density of ice is less than water. So, the buoyant force will balance out the force of. Explain Why Ice Is Less Dense Than Water.
From slideplayer.com
Day ppt download Explain Why Ice Is Less Dense Than Water Ice is less dense than liquid water and so it floats. If you put pressure on regular ice, and give it time to rearrange, the molecules will move into a. Hydrogen bonds hold water molecules in place in solid phase. The density of ice, on the other hand, is about 0.9 g/cm^3. So, even though water is more dense than. Explain Why Ice Is Less Dense Than Water.
From juniorferslamb.blogspot.com
Why is Ice Less Dense Than Water Explain Why Ice Is Less Dense Than Water It is less dense as a solid than as a liquid, because its particles move apart slightly on freezing. The density of ice, on the other hand, is about 0.9 g/cm^3. If you put pressure on regular ice, and give it time to rearrange, the molecules will move into a. So, the buoyant force will balance out the force of. Explain Why Ice Is Less Dense Than Water.
From www.chegg.com
Solved 3. Ice is less dense than water, which is why ice Explain Why Ice Is Less Dense Than Water Hydrogen bonds hold water molecules in place in solid phase. So, even though water is more dense than ice, ice still. The density of ice is less than water. The density of ice, on the other hand, is about 0.9 g/cm^3. The density of water is about 1 gram per cubic centimeter (g/cm^3). It is less dense as a solid. Explain Why Ice Is Less Dense Than Water.
From 9to5science.com
[Solved] Why is ice less dense than water? 9to5Science Explain Why Ice Is Less Dense Than Water All of this physics comes. The density of ice, on the other hand, is about 0.9 g/cm^3. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. So, even though water is more dense than ice, ice still. If you put pressure on regular ice, and give it time. Explain Why Ice Is Less Dense Than Water.
From nicholasbsullivan.com
Images and Figures for Oceanography Explain Why Ice Is Less Dense Than Water Yes, some ice is denser than water. Ponds or lakes begin to freeze at the surface, closer to the cold air. The density of ice is less than water. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. It is less dense as a solid than as a liquid, because. Explain Why Ice Is Less Dense Than Water.
From www.dreamstime.com
Why Does Ice Float on Water Infographic Diagram Stock Vector Explain Why Ice Is Less Dense Than Water A layer of ice forms, but does not sink as it would if water did not have this unique structure dictated by its shape, polarity, and hydrogen bonding. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. Ice is less denser than water because in ice the molecules arrange themselves. Explain Why Ice Is Less Dense Than Water.
From www.slideserve.com
PPT Chapter 3 Chemical and Physical Features of Seawater PowerPoint Explain Why Ice Is Less Dense Than Water Ice is less dense than liquid water and so it floats. A layer of ice forms, but does not sink as it would if water did not have this unique structure dictated by its shape, polarity, and hydrogen bonding. Hydrogen bonds hold water molecules in place in solid phase. It is less dense as a solid than as a liquid,. Explain Why Ice Is Less Dense Than Water.
From www.youtube.com
Why is ice less dense than water YouTube Explain Why Ice Is Less Dense Than Water Water is different from most substances. Yes, some ice is denser than water. The density of water is about 1 gram per cubic centimeter (g/cm^3). All of this physics comes. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. Ice is less dense than liquid water and so it floats.. Explain Why Ice Is Less Dense Than Water.
From www.expii.com
Density of Water — Comparison of Solid vs. Liquid Expii Explain Why Ice Is Less Dense Than Water The density of water is about 1 gram per cubic centimeter (g/cm^3). Structure of ice is a regular open framework of water molecules arranged like honeycomb. Ice is less dense than liquid water and so it floats. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of. Explain Why Ice Is Less Dense Than Water.
From www.slideserve.com
PPT Physical Properties of Water PowerPoint Presentation, free Explain Why Ice Is Less Dense Than Water Ice is less dense than liquid water and so it floats. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. So, even though water is more dense than ice, ice still. Water is different from most substances. Structure of ice is a regular open framework of water molecules arranged like. Explain Why Ice Is Less Dense Than Water.
From www.slideserve.com
PPT LIFE DEPENDS ON THE UNIQUE PROPERITIES OF WATER PowerPoint Explain Why Ice Is Less Dense Than Water The density of water is about 1 gram per cubic centimeter (g/cm^3). Ponds or lakes begin to freeze at the surface, closer to the cold air. Structure of ice is a regular open framework of water molecules arranged like honeycomb. A layer of ice forms, but does not sink as it would if water did not have this unique structure. Explain Why Ice Is Less Dense Than Water.
From www.slideserve.com
PPT The Extraordinary Properties of Water PowerPoint Presentation Explain Why Ice Is Less Dense Than Water The density of ice, on the other hand, is about 0.9 g/cm^3. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. Ice is less dense than liquid water and so it floats. This is due to the water expanding as it is frozen because of the hydrogen forming. Explain Why Ice Is Less Dense Than Water.
From juniorferslamb.blogspot.com
Why is Ice Less Dense Than Water Explain Why Ice Is Less Dense Than Water Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. So, even though water is more dense than ice, ice still. This is due to the water expanding as it is frozen because of the hydrogen forming an open type. If you put pressure on regular ice, and give. Explain Why Ice Is Less Dense Than Water.
From uhighlsu.web.fc2.com
is ice less dense than water Explain Why Ice Is Less Dense Than Water The density of ice is less than water. Water is different from most substances. Ice is less denser than water because in ice the molecules arrange themselves in a rigid tetrahedral structure due to which. So, even though water is more dense than ice, ice still. The density of ice, on the other hand, is about 0.9 g/cm^3. A layer. Explain Why Ice Is Less Dense Than Water.
From www.slideserve.com
PPT Understanding Water PowerPoint Presentation ID2739092 Explain Why Ice Is Less Dense Than Water The density of ice, on the other hand, is about 0.9 g/cm^3. The density of water is about 1 gram per cubic centimeter (g/cm^3). So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. This is due to the water expanding as it is frozen because. Explain Why Ice Is Less Dense Than Water.