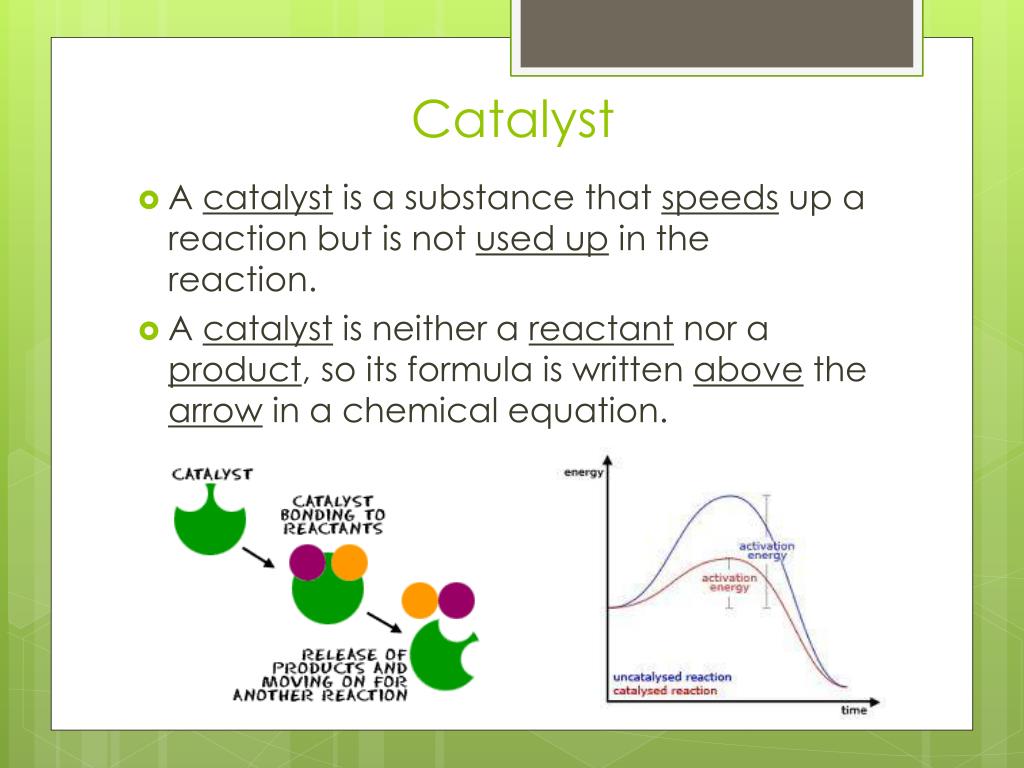
Example Of A Catalyst . In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Most examples of heterogeneous catalysis go through the same stages: Also, find out how catalysts can be used figuratively to describe social or personal changes. There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. One or more of the. Typical examples involve a solid catalyst with the reactants as either liquids or gases. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids.
from www.slideserve.com
Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. Most examples of heterogeneous catalysis go through the same stages: In a heterogeneous reaction, the catalyst is in a different phase from the reactants. In general, catalytic action is a chemical reaction between the catalyst and a reactant. One or more of the. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Typical examples involve a solid catalyst with the reactants as either liquids or gases. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Also, find out how catalysts can be used figuratively to describe social or personal changes. There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur.
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free
Example Of A Catalyst Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. Also, find out how catalysts can be used figuratively to describe social or personal changes. Most examples of heterogeneous catalysis go through the same stages: Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Typical examples involve a solid catalyst with the reactants as either liquids or gases. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. One or more of the. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur.
From www.nagwa.com
Lesson Catalysts Nagwa Example Of A Catalyst Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. One or more of the. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. Most examples of heterogeneous catalysis go through the same stages: Also, find out how catalysts can be used figuratively to describe social or personal. Example Of A Catalyst.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Example Of A Catalyst Also, find out how catalysts can be used figuratively to describe social or personal changes. One or more of the. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Typical examples involve a solid catalyst with the reactants as either liquids or gases. There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction. Example Of A Catalyst.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Example Of A Catalyst Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. Most examples of. Example Of A Catalyst.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Example Of A Catalyst Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Also, find out how catalysts can be used figuratively to describe social or personal changes. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. Most examples of heterogeneous catalysis go through the same stages: Typical examples involve a solid. Example Of A Catalyst.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Example Of A Catalyst In general, catalytic action is a chemical reaction between the catalyst and a reactant. Typical examples involve a solid catalyst with the reactants as either liquids or gases. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. Also, find out how catalysts can be used figuratively to describe social or personal changes.. Example Of A Catalyst.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Example Of A Catalyst Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Most examples of heterogeneous catalysis go through the same stages: Also, find out how catalysts can be used figuratively to describe social or personal changes. In a. Example Of A Catalyst.
From www.slideshare.net
Catalysis Example Of A Catalyst In general, catalytic action is a chemical reaction between the catalyst and a reactant. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn what a catalyst is. Example Of A Catalyst.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Example Of A Catalyst Most examples of heterogeneous catalysis go through the same stages: One or more of the. Also, find out how catalysts can be used figuratively to describe social or personal changes. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn what a catalyst is and see examples of chemical catalysts such as metals,. Example Of A Catalyst.
From www.youtube.com
How does a CATALYST work ? YouTube Example Of A Catalyst Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Most examples of heterogeneous catalysis go through the same stages: In a heterogeneous reaction, the catalyst is in a different phase from the reactants. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Learn what a catalyst is and. Example Of A Catalyst.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 Example Of A Catalyst Typical examples involve a solid catalyst with the reactants as either liquids or gases. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Also, find out how catalysts can be used figuratively to describe social or personal changes. One or more of the. Enzymes are naturally occurring catalysts responsible for many essential biochemical. Example Of A Catalyst.
From www.youtube.com
Catalysts Chemistry Khan Academy YouTube Example Of A Catalyst Also, find out how catalysts can be used figuratively to describe social or personal changes. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. There are also negative. Example Of A Catalyst.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Example Of A Catalyst There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur. Most examples of heterogeneous catalysis go through the same stages: In a heterogeneous reaction, the catalyst is in a different phase from the reactants. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself. Example Of A Catalyst.
From www.slideshare.net
Catalyst Example Of A Catalyst One or more of the. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Also, find out how catalysts can be used figuratively to describe social or personal changes. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. Catalyst, in chemistry, any substance that increases the rate. Example Of A Catalyst.
From www.thoughtco.com
Catalysis Definition in Chemistry Example Of A Catalyst One or more of the. There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. Most examples of. Example Of A Catalyst.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Example Of A Catalyst Also, find out how catalysts can be used figuratively to describe social or personal changes. Typical examples involve a solid catalyst with the reactants as either liquids or gases. Most examples of heterogeneous catalysis go through the same stages: Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Usually when someone refers to a catalyst, they mean a. Example Of A Catalyst.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Example Of A Catalyst Also, find out how catalysts can be used figuratively to describe social or personal changes. Most examples of heterogeneous catalysis go through the same stages: In a heterogeneous reaction, the catalyst is in a different phase from the reactants. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Typical examples involve a solid catalyst with. Example Of A Catalyst.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Example Of A Catalyst In general, catalytic action is a chemical reaction between the catalyst and a reactant. Typical examples involve a solid catalyst with the reactants as either liquids or gases. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. Enzymes are. Example Of A Catalyst.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Example Of A Catalyst Typical examples involve a solid catalyst with the reactants as either liquids or gases. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. Also, find out how catalysts can be used figuratively to describe social or personal changes. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Usually when someone refers to a. Example Of A Catalyst.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Example Of A Catalyst In a heterogeneous reaction, the catalyst is in a different phase from the reactants. Most examples of heterogeneous catalysis go through the same stages: Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. Catalyst, in chemistry, any substance that. Example Of A Catalyst.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Example Of A Catalyst Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. One or more of the. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. There are also negative catalysts or inhibitors, which slow the rate of a. Example Of A Catalyst.
From joilxpqmt.blob.core.windows.net
Chemical Catalysis Examples at Howard Wade blog Example Of A Catalyst Typical examples involve a solid catalyst with the reactants as either liquids or gases. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. Also, find out how. Example Of A Catalyst.
From study.com
Catalyst Definition, Types & Function Lesson Example Of A Catalyst Typical examples involve a solid catalyst with the reactants as either liquids or gases. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. In general,. Example Of A Catalyst.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Example Of A Catalyst In a heterogeneous reaction, the catalyst is in a different phase from the reactants. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Also, find out how catalysts can be used figuratively to describe social or personal changes. Most examples of heterogeneous catalysis go through the same stages: Catalyst, in chemistry, any substance that increases the rate of. Example Of A Catalyst.
From joifdozvf.blob.core.windows.net
What Is A Catalyst In Chemistry Example at Herbert Simpson blog Example Of A Catalyst Typical examples involve a solid catalyst with the reactants as either liquids or gases. Most examples of heterogeneous catalysis go through the same stages: In general, catalytic action is a chemical reaction between the catalyst and a reactant. One or more of the. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids.. Example Of A Catalyst.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2823635 Example Of A Catalyst In a heterogeneous reaction, the catalyst is in a different phase from the reactants. Also, find out how catalysts can be used figuratively to describe social or personal changes. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes. Example Of A Catalyst.
From www.catalystseurope.org
How are catalysts used? Example Of A Catalyst Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. One or more of the. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Most examples of heterogeneous catalysis go through the same. Example Of A Catalyst.
From www.slideshare.net
Catalysis Example Of A Catalyst There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur. Most examples of heterogeneous catalysis go through the same stages: Also, find out how catalysts can be used figuratively to describe social or personal changes. Typical examples involve a solid catalyst with the reactants as either liquids or. Example Of A Catalyst.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Example Of A Catalyst Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalyst, in chemistry, any. Example Of A Catalyst.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Example Of A Catalyst Most examples of heterogeneous catalysis go through the same stages: In general, catalytic action is a chemical reaction between the catalyst and a reactant. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. In a heterogeneous reaction, the catalyst. Example Of A Catalyst.
From www.slideserve.com
PPT Basic Chemistry Concepts PowerPoint Presentation, free download Example Of A Catalyst There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Typical examples involve a solid catalyst with the reactants as either liquids or gases. Enzymes are naturally occurring catalysts responsible for many essential biochemical. Example Of A Catalyst.
From scitechdaily.com
Science Made Simple What Are Catalysts? Example Of A Catalyst Typical examples involve a solid catalyst with the reactants as either liquids or gases. Most examples of heterogeneous catalysis go through the same stages: In general, catalytic action is a chemical reaction between the catalyst and a reactant. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. One or more of the. In a heterogeneous reaction, the catalyst. Example Of A Catalyst.
From www.slideshare.net
Biology 2.4 Example Of A Catalyst There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely to occur. Most examples of heterogeneous catalysis go through the same stages: Typical examples involve a solid catalyst with the reactants as either liquids or gases. Also, find out how catalysts can be used figuratively to describe social or personal. Example Of A Catalyst.
From exomdmjjp.blob.core.windows.net
Examples Of Catalysts Bbc Bitesize at Jacqueline Epp blog Example Of A Catalyst Learn what a catalyst is and see examples of chemical catalysts such as metals, enzymes and acids. Also, find out how catalysts can be used figuratively to describe social or personal changes. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its. Example Of A Catalyst.
From joilxpqmt.blob.core.windows.net
Chemical Catalysis Examples at Howard Wade blog Example Of A Catalyst In general, catalytic action is a chemical reaction between the catalyst and a reactant. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Also, find out how catalysts can be used figuratively to describe social or personal changes. There are also negative catalysts or inhibitors, which slow the rate of a chemical reaction or make it less likely. Example Of A Catalyst.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Example Of A Catalyst In a heterogeneous reaction, the catalyst is in a different phase from the reactants. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation energy. Typical examples involve a solid catalyst with the reactants as either liquids or gases. Most examples. Example Of A Catalyst.