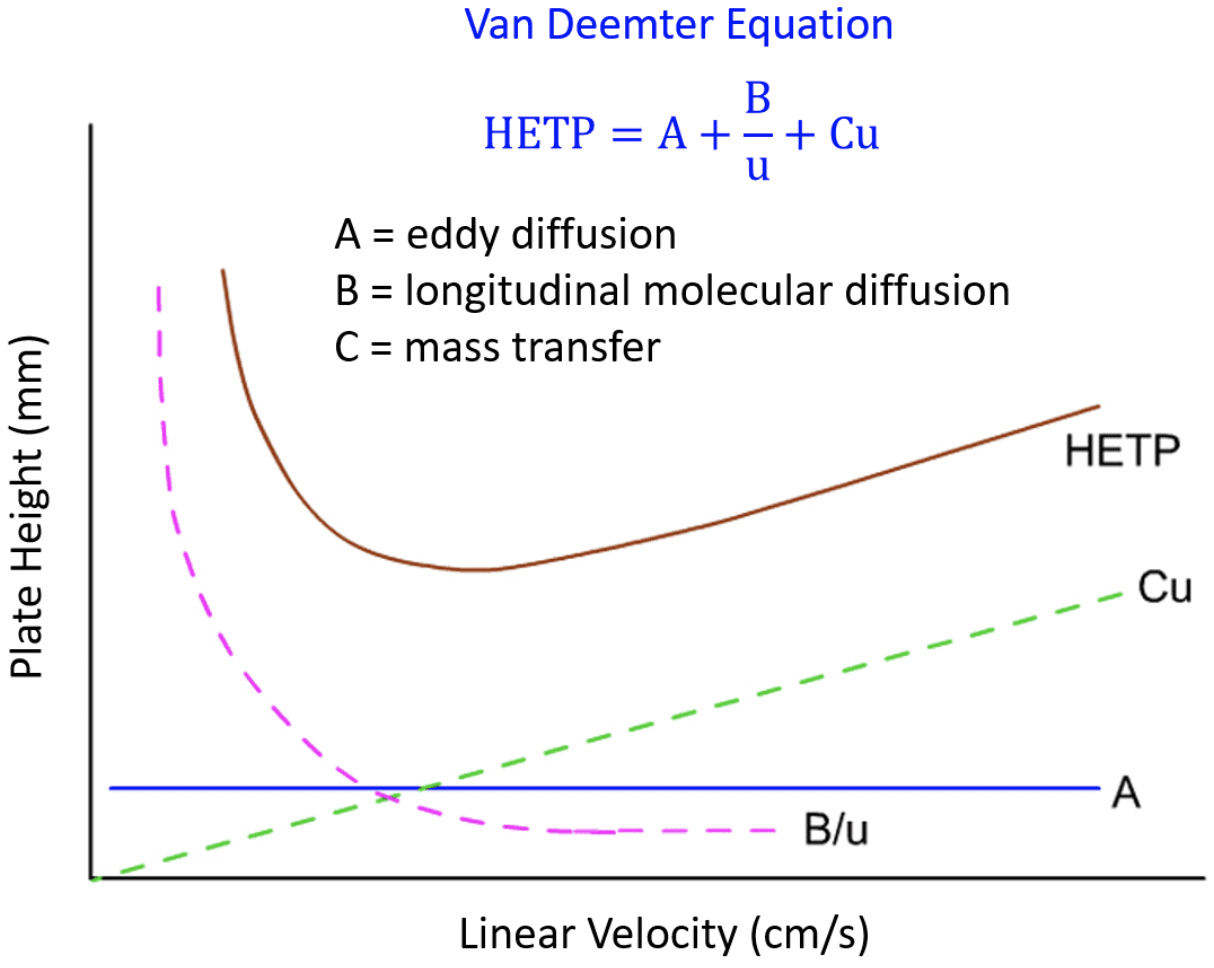
Gas Chromatography Van Deemter Equation . gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas phase. what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the contribution of longitudinal diffusion. the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the.
from www.crawfordscientific.com
gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas phase. what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the contribution of longitudinal diffusion. this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the.
Van Deemter equation The Lockdown guide
Gas Chromatography Van Deemter Equation the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the contribution of longitudinal diffusion. what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas phase. the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors.
From www.numerade.com
SOLVED The Van Deemter equation is governed by three terms multiple Gas Chromatography Van Deemter Equation gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas phase. what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for. Gas Chromatography Van Deemter Equation.
From www.slideserve.com
PPT Ch 21 Principles of Chromatography and Mass Spectrometry Ch 22 Gas Chromatography Van Deemter Equation in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the contribution of longitudinal diffusion. gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas phase. the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the. Gas Chromatography Van Deemter Equation.
From www.slideserve.com
PPT GAS CHROMATOGRAPHY PowerPoint Presentation, free download ID Gas Chromatography Van Deemter Equation the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the contribution of longitudinal diffusion. what we will develop as we analyze the four contributions to broadening above is an. Gas Chromatography Van Deemter Equation.
From www.slideserve.com
PPT Van Deemter Equation PowerPoint Presentation, free download ID Gas Chromatography Van Deemter Equation the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas phase. what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the. Gas Chromatography Van Deemter Equation.
From www.slideshare.net
gas chromatography (GC) Gas Chromatography Van Deemter Equation what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. gas chromatography separates gaseous substances based on partitioning in a stationary phase. Gas Chromatography Van Deemter Equation.
From pubs.acs.org
Modifications to the van Deemter Equation for the Height Equivalent to Gas Chromatography Van Deemter Equation the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the contribution of longitudinal diffusion. what we will develop as we analyze the four contributions to broadening above is an. Gas Chromatography Van Deemter Equation.
From www.coleparmer.de
Science of Chromatography Gas Chromatography Van Deemter Equation gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas phase. the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the.. Gas Chromatography Van Deemter Equation.
From www.researchgate.net
Plate height (H) versus flow rate using Van Deemter equation Download Gas Chromatography Van Deemter Equation gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas phase. what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on. Gas Chromatography Van Deemter Equation.
From www.semanticscholar.org
Figure 1 from Modifications to the van Deemter Equation in Gas Gas Chromatography Van Deemter Equation this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the contribution of longitudinal diffusion. the van deemter equation is the basic interpretive equation of column efficiency,. Gas Chromatography Van Deemter Equation.
From www.youtube.com
Van Deemter equation Chromatography gas chromatography Hplc Gas Chromatography Van Deemter Equation what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the contribution of longitudinal diffusion. the van deemter equation is the basic interpretive equation of column. Gas Chromatography Van Deemter Equation.
From www.youtube.com
Chromatography Van Deemter equation Gas chromatography HPLC Gas Chromatography Van Deemter Equation the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. in the van deemter equation, a accounts for the contribution of multiple paths (h p),. Gas Chromatography Van Deemter Equation.
From www.slideshare.net
gas chromatography (GC) Gas Chromatography Van Deemter Equation this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas phase. the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors.. Gas Chromatography Van Deemter Equation.
From www.scribd.com
Van Deemter Chromatography Gas Chromatography Gas Chromatography Van Deemter Equation the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the contribution of longitudinal diffusion. gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas. Gas Chromatography Van Deemter Equation.
From www.slideshare.net
gas chromatography (GC) Gas Chromatography Van Deemter Equation in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the contribution of longitudinal diffusion. this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. the van deemter equation is the basic interpretive equation of column efficiency,. Gas Chromatography Van Deemter Equation.
From yourcolumninfo.blogspot.com
Van Deemter equation Gas Chromatography Van Deemter Equation what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. gas chromatography separates gaseous substances based on partitioning in a stationary phase. Gas Chromatography Van Deemter Equation.
From www.chegg.com
Solved To the right is a plot of the van Deemter equation, Gas Chromatography Van Deemter Equation this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas phase. in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the. Gas Chromatography Van Deemter Equation.
From dokumen.tips
(PDF) Gas Chromatography Fundamentals Theory · 6/2/2018 · Van Deemter Gas Chromatography Van Deemter Equation the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. this equation shows the relationship between peak efficiency and linear velocity and please see the. Gas Chromatography Van Deemter Equation.
From handwiki.org
Van Deemter equation HandWiki Gas Chromatography Van Deemter Equation this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. what we will develop as we analyze the four contributions to broadening above is an equation,. Gas Chromatography Van Deemter Equation.
From www.numerade.com
The van Deemter curves for gas chromatography of hexadecane, CH, using Gas Chromatography Van Deemter Equation the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the contribution of longitudinal diffusion. what we will develop as we analyze the four contributions to broadening above is an. Gas Chromatography Van Deemter Equation.
From www.youtube.com
Theories in Chromatography Part 3_Rate Theory Van Deemter equation Gas Chromatography Van Deemter Equation what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. in the van deemter equation, a accounts for the contribution of multiple paths (h p),. Gas Chromatography Van Deemter Equation.
From www.slideserve.com
PPT Gas Chromatography PowerPoint Presentation, free download ID Gas Chromatography Van Deemter Equation this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. in the van deemter equation, a accounts for the contribution of multiple. Gas Chromatography Van Deemter Equation.
From www.scribd.com
Van Deemter Equation PDF Chromatography High Performance Liquid Gas Chromatography Van Deemter Equation gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas phase. the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the.. Gas Chromatography Van Deemter Equation.
From www.researchgate.net
Van Deemter equation describes that efficiency varies with the linear Gas Chromatography Van Deemter Equation in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the contribution of longitudinal diffusion. this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. the van deemter equation is the basic interpretive equation of column efficiency,. Gas Chromatography Van Deemter Equation.
From www.scribd.com
A. Van Deemter Equation in Chromatography. Solution PDF Gas Chromatography Van Deemter Equation the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. in the van deemter equation, a accounts for the contribution of multiple paths (h p),. Gas Chromatography Van Deemter Equation.
From slidetodoc.com
Fundamentals of Gas Chromatography Theory BUILDING BETTER SCIENCE Gas Chromatography Van Deemter Equation the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas phase.. Gas Chromatography Van Deemter Equation.
From www.crawfordscientific.com
Van Deemter equation The Lockdown guide Gas Chromatography Van Deemter Equation the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. this equation shows the relationship between peak efficiency and linear velocity and please see the. Gas Chromatography Van Deemter Equation.
From slidetodoc.com
Chromatography Introduction GENERAL THEORY OF COLUMN CHROMATOGRAPHY Gas Chromatography Van Deemter Equation in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the contribution of longitudinal diffusion. this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. the van deemter equation is the basic interpretive equation of column efficiency,. Gas Chromatography Van Deemter Equation.
From www.researchgate.net
Representación gráfica de la ecuación de van Deemter. En el inserto Gas Chromatography Van Deemter Equation this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas phase. in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the. Gas Chromatography Van Deemter Equation.
From slidetodoc.com
Fundamentals of Gas Chromatography Theory BUILDING BETTER SCIENCE Gas Chromatography Van Deemter Equation this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. in the van deemter equation, a accounts for the contribution of multiple. Gas Chromatography Van Deemter Equation.
From www.slideserve.com
PPT Schematic of a GC PowerPoint Presentation, free download ID351780 Gas Chromatography Van Deemter Equation this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u. Gas Chromatography Van Deemter Equation.
From www.numerade.com
SOLVED Consider the use of the capillary column in gas chromatography Gas Chromatography Van Deemter Equation the van deemter equation is the basic interpretive equation of column efficiency, which provides an insight into the factors. this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas phase.. Gas Chromatography Van Deemter Equation.
From www.barts-blog.net
As Easy as ABC How to Use the Van Deemter Equation to Optimize Your Gas Chromatography Van Deemter Equation in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the contribution of longitudinal diffusion. gas chromatography separates gaseous substances based on partitioning in a stationary phase from a gas phase. what we will develop as we analyze the four contributions to broadening above is an equation, which was. Gas Chromatography Van Deemter Equation.
From www.slideserve.com
PPT Van Deemter Equation PowerPoint Presentation, free download ID Gas Chromatography Van Deemter Equation this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. the van deemter equation is the basic interpretive equation of column efficiency,. Gas Chromatography Van Deemter Equation.
From www.scientistlive.com
Alternatives to helium Scientist Live Gas Chromatography Van Deemter Equation what we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van. this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. gas chromatography separates gaseous substances based on partitioning in a stationary phase. Gas Chromatography Van Deemter Equation.
From www.youtube.com
Van deemter equation YouTube Gas Chromatography Van Deemter Equation this equation shows the relationship between peak efficiency and linear velocity and please see the chromacademy module on band broadening for the. in the van deemter equation, a accounts for the contribution of multiple paths (h p), b/u accounts for the contribution of longitudinal diffusion. what we will develop as we analyze the four contributions to broadening. Gas Chromatography Van Deemter Equation.