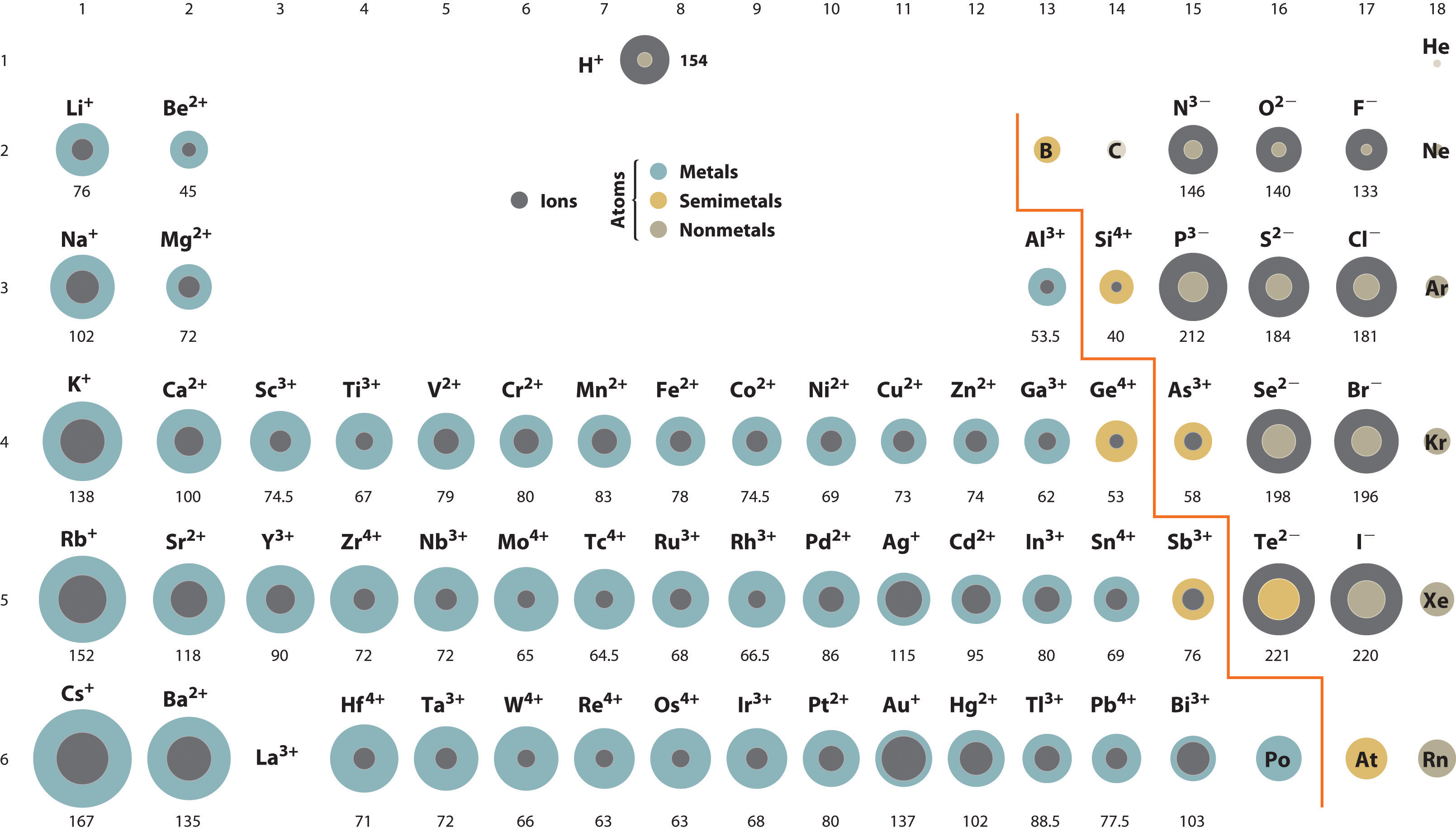
Are All Atoms Same Size . Atoms of a given element are identical in size, mass, and other properties. Atoms cannot be subdivided, created, or destroyed. Each atom’s size is relative to the largest element, cesium. All matter is composed of extremely small particles called atoms. What makes atoms of different elements different? This is because each atom further down the column has more protons and neutrons and also gains an. All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are particles. As you move down an element group (column), the size of atoms increases. This periodic table shows the relative sizes of the atoms of each element. The fundamental characteristic that all atoms of the same. Atoms of different elements differ in size, mass, and other properties.
from chem.libretexts.org
As you move down an element group (column), the size of atoms increases. The fundamental characteristic that all atoms of the same. All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are particles. Atoms of a given element are identical in size, mass, and other properties. All matter is composed of extremely small particles called atoms. Atoms of different elements differ in size, mass, and other properties. Atoms cannot be subdivided, created, or destroyed. This is because each atom further down the column has more protons and neutrons and also gains an. Each atom’s size is relative to the largest element, cesium. This periodic table shows the relative sizes of the atoms of each element.
7.3 Sizes of Atoms and Ions Chemistry LibreTexts
Are All Atoms Same Size Atoms cannot be subdivided, created, or destroyed. Atoms cannot be subdivided, created, or destroyed. All matter is composed of extremely small particles called atoms. What makes atoms of different elements different? The fundamental characteristic that all atoms of the same. This is because each atom further down the column has more protons and neutrons and also gains an. Each atom’s size is relative to the largest element, cesium. This periodic table shows the relative sizes of the atoms of each element. All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are particles. Atoms of different elements differ in size, mass, and other properties. Atoms of a given element are identical in size, mass, and other properties. As you move down an element group (column), the size of atoms increases.
From www.teachoo.com
What is Atom? How does it Exist? and it's Symbols Teachoo Are All Atoms Same Size This is because each atom further down the column has more protons and neutrons and also gains an. Atoms of a given element are identical in size, mass, and other properties. Atoms cannot be subdivided, created, or destroyed. All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are particles. Atoms. Are All Atoms Same Size.
From bramblechemistry.weebly.com
1B3 Atoms and Molecules Are All Atoms Same Size The fundamental characteristic that all atoms of the same. Atoms of a given element are identical in size, mass, and other properties. What makes atoms of different elements different? All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are particles. Each atom’s size is relative to the largest element, cesium.. Are All Atoms Same Size.
From www.yourdictionary.com
Basic Difference Between an Atom and a Molecule YourDictionary Are All Atoms Same Size All matter is composed of extremely small particles called atoms. Atoms cannot be subdivided, created, or destroyed. What makes atoms of different elements different? Atoms of different elements differ in size, mass, and other properties. As you move down an element group (column), the size of atoms increases. All atoms are made from the same bits, which are called subatomic. Are All Atoms Same Size.
From www.slideserve.com
PPT Chapter 38 The Atom and the Quantum PowerPoint Presentation, free download ID1226743 Are All Atoms Same Size This is because each atom further down the column has more protons and neutrons and also gains an. All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are particles. Atoms of different elements differ in size, mass, and other properties. Atoms of a given element are identical in size, mass,. Are All Atoms Same Size.
From www.slideserve.com
PPT Many electron atoms and the Periodic Table PowerPoint Presentation ID6900803 Are All Atoms Same Size Atoms of different elements differ in size, mass, and other properties. The fundamental characteristic that all atoms of the same. All matter is composed of extremely small particles called atoms. Each atom’s size is relative to the largest element, cesium. This periodic table shows the relative sizes of the atoms of each element. Atoms of a given element are identical. Are All Atoms Same Size.
From chem.libretexts.org
7.3 Sizes of Atoms and Ions Chemistry LibreTexts Are All Atoms Same Size All matter is composed of extremely small particles called atoms. What makes atoms of different elements different? Atoms cannot be subdivided, created, or destroyed. Atoms of a given element are identical in size, mass, and other properties. This periodic table shows the relative sizes of the atoms of each element. This is because each atom further down the column has. Are All Atoms Same Size.
From courses.lumenlearning.com
Periodic Variations in Element Properties CHEM 1305 General Chemistry I—Lecture Are All Atoms Same Size All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are particles. Atoms of a given element are identical in size, mass, and other properties. Atoms of different elements differ in size, mass, and other properties. Atoms cannot be subdivided, created, or destroyed. This is because each atom further down the. Are All Atoms Same Size.
From payscalechart.z28.web.core.windows.net
atomic scale chart Table periodic chemistry elements chemicool Are All Atoms Same Size What makes atoms of different elements different? All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are particles. This is because each atom further down the column has more protons and neutrons and also gains an. Atoms cannot be subdivided, created, or destroyed. Each atom’s size is relative to the. Are All Atoms Same Size.
From www.slideserve.com
PPT ATOMS & THE PERIODIC TABLE PowerPoint Presentation, free download ID6806231 Are All Atoms Same Size Each atom’s size is relative to the largest element, cesium. All matter is composed of extremely small particles called atoms. What makes atoms of different elements different? Atoms of different elements differ in size, mass, and other properties. This periodic table shows the relative sizes of the atoms of each element. Atoms of a given element are identical in size,. Are All Atoms Same Size.
From hubpages.com
Atoms and Atomic Structure HubPages Are All Atoms Same Size Atoms of a given element are identical in size, mass, and other properties. What makes atoms of different elements different? This is because each atom further down the column has more protons and neutrons and also gains an. Each atom’s size is relative to the largest element, cesium. All atoms are made from the same bits, which are called subatomic. Are All Atoms Same Size.
From spmscience.blog.onlinetuition.com.my
4.2 Structure of Atoms SPM Science Are All Atoms Same Size All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are particles. Each atom’s size is relative to the largest element, cesium. All matter is composed of extremely small particles called atoms. Atoms cannot be subdivided, created, or destroyed. Atoms of different elements differ in size, mass, and other properties. This. Are All Atoms Same Size.
From chemistry.about.com
Basic Model of the Atom Atomic Theory Are All Atoms Same Size This is because each atom further down the column has more protons and neutrons and also gains an. As you move down an element group (column), the size of atoms increases. Atoms of different elements differ in size, mass, and other properties. All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and. Are All Atoms Same Size.
From www.chem.fsu.edu
Electron Configurations Are All Atoms Same Size The fundamental characteristic that all atoms of the same. What makes atoms of different elements different? Atoms of different elements differ in size, mass, and other properties. This periodic table shows the relative sizes of the atoms of each element. As you move down an element group (column), the size of atoms increases. Each atom’s size is relative to the. Are All Atoms Same Size.
From chemwiki.ucdavis.edu
Chapter 3.2 Sizes of Atoms and Ions Chemwiki Are All Atoms Same Size All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are particles. The fundamental characteristic that all atoms of the same. Atoms of different elements differ in size, mass, and other properties. This is because each atom further down the column has more protons and neutrons and also gains an. Atoms. Are All Atoms Same Size.
From www.teachoo.com
What is Atom? How does it Exist? and it's Symbols Teachoo Are All Atoms Same Size All matter is composed of extremely small particles called atoms. Each atom’s size is relative to the largest element, cesium. This periodic table shows the relative sizes of the atoms of each element. What makes atoms of different elements different? Atoms cannot be subdivided, created, or destroyed. Atoms of a given element are identical in size, mass, and other properties.. Are All Atoms Same Size.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Are All Atoms Same Size This is because each atom further down the column has more protons and neutrons and also gains an. Each atom’s size is relative to the largest element, cesium. Atoms of different elements differ in size, mass, and other properties. This periodic table shows the relative sizes of the atoms of each element. What makes atoms of different elements different? The. Are All Atoms Same Size.
From www.sciencefacts.net
Atom Definition, Structure & Parts with Labeled Diagram Are All Atoms Same Size Atoms of different elements differ in size, mass, and other properties. This periodic table shows the relative sizes of the atoms of each element. Each atom’s size is relative to the largest element, cesium. All matter is composed of extremely small particles called atoms. This is because each atom further down the column has more protons and neutrons and also. Are All Atoms Same Size.
From courses.lumenlearning.com
Atomic Size Introduction to Chemistry Are All Atoms Same Size All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are particles. Atoms cannot be subdivided, created, or destroyed. The fundamental characteristic that all atoms of the same. As you move down an element group (column), the size of atoms increases. This periodic table shows the relative sizes of the atoms. Are All Atoms Same Size.
From democracyunlimited.web.fc2.com
are all atoms the same Are All Atoms Same Size Atoms of a given element are identical in size, mass, and other properties. The fundamental characteristic that all atoms of the same. Each atom’s size is relative to the largest element, cesium. Atoms of different elements differ in size, mass, and other properties. Atoms cannot be subdivided, created, or destroyed. What makes atoms of different elements different? All atoms are. Are All Atoms Same Size.
From www.teachoo.com
Nucleons, Atomic Number and Mass Number Definition [with Examples] Are All Atoms Same Size Atoms cannot be subdivided, created, or destroyed. As you move down an element group (column), the size of atoms increases. Each atom’s size is relative to the largest element, cesium. All matter is composed of extremely small particles called atoms. All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are. Are All Atoms Same Size.
From slideplayer.com
Chemical Periodicity Chapter ppt download Are All Atoms Same Size Each atom’s size is relative to the largest element, cesium. This periodic table shows the relative sizes of the atoms of each element. What makes atoms of different elements different? As you move down an element group (column), the size of atoms increases. All matter is composed of extremely small particles called atoms. This is because each atom further down. Are All Atoms Same Size.
From askfilo.com
3. All the atoms of a same element have identical mass and identical chem.. Are All Atoms Same Size All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are particles. The fundamental characteristic that all atoms of the same. Atoms of different elements differ in size, mass, and other properties. This is because each atom further down the column has more protons and neutrons and also gains an. Each. Are All Atoms Same Size.
From study.com
What are Atoms & Molecules? Definition & Differences Video & Lesson Transcript Are All Atoms Same Size All matter is composed of extremely small particles called atoms. Atoms of a given element are identical in size, mass, and other properties. Atoms cannot be subdivided, created, or destroyed. All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are particles. The fundamental characteristic that all atoms of the same.. Are All Atoms Same Size.
From saylordotorg.github.io
Sizes of Atoms and Ions Are All Atoms Same Size Atoms cannot be subdivided, created, or destroyed. Atoms of different elements differ in size, mass, and other properties. This periodic table shows the relative sizes of the atoms of each element. Each atom’s size is relative to the largest element, cesium. All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these. Are All Atoms Same Size.
From www.slideserve.com
PPT Atoms The Building Blocks of Matter PowerPoint Presentation, free download ID6205752 Are All Atoms Same Size As you move down an element group (column), the size of atoms increases. This is because each atom further down the column has more protons and neutrons and also gains an. The fundamental characteristic that all atoms of the same. Atoms of a given element are identical in size, mass, and other properties. Atoms of different elements differ in size,. Are All Atoms Same Size.
From www.youtube.com
Atom Size Comparison 4K YouTube Are All Atoms Same Size Atoms of a given element are identical in size, mass, and other properties. The fundamental characteristic that all atoms of the same. As you move down an element group (column), the size of atoms increases. What makes atoms of different elements different? Each atom’s size is relative to the largest element, cesium. All atoms are made from the same bits,. Are All Atoms Same Size.
From www.youtube.com
How Big are Atoms (Size Comparison) Atoms and Molecules 5 in Hindi for Class 9 Science Are All Atoms Same Size Atoms cannot be subdivided, created, or destroyed. This periodic table shows the relative sizes of the atoms of each element. What makes atoms of different elements different? The fundamental characteristic that all atoms of the same. All matter is composed of extremely small particles called atoms. All atoms are made from the same bits, which are called subatomic particles (sub. Are All Atoms Same Size.
From sciencenotes.org
Atomic Radius and Ionic Radius Are All Atoms Same Size Atoms cannot be subdivided, created, or destroyed. What makes atoms of different elements different? This is because each atom further down the column has more protons and neutrons and also gains an. Atoms of different elements differ in size, mass, and other properties. The fundamental characteristic that all atoms of the same. All matter is composed of extremely small particles. Are All Atoms Same Size.
From www.sciencefacts.net
Atom Definition, Structure & Parts with Labeled Diagram Are All Atoms Same Size Atoms of different elements differ in size, mass, and other properties. This is because each atom further down the column has more protons and neutrons and also gains an. Atoms of a given element are identical in size, mass, and other properties. This periodic table shows the relative sizes of the atoms of each element. Atoms cannot be subdivided, created,. Are All Atoms Same Size.
From sciencenotes.org
Learn the Parts of an Atom Are All Atoms Same Size Atoms of a given element are identical in size, mass, and other properties. The fundamental characteristic that all atoms of the same. This is because each atom further down the column has more protons and neutrons and also gains an. All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are. Are All Atoms Same Size.
From www.thedailyeco.com
The Differences Between Atoms and Molecules Atom and Molecule Comparison With Diagrams Are All Atoms Same Size This periodic table shows the relative sizes of the atoms of each element. What makes atoms of different elements different? Atoms of different elements differ in size, mass, and other properties. All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are particles. Atoms of a given element are identical in. Are All Atoms Same Size.
From sciencenotes.org
Are Two Atoms of the Same Element Identical? Are All Atoms Same Size What makes atoms of different elements different? This periodic table shows the relative sizes of the atoms of each element. The fundamental characteristic that all atoms of the same. Atoms of a given element are identical in size, mass, and other properties. Atoms of different elements differ in size, mass, and other properties. All atoms are made from the same. Are All Atoms Same Size.
From chem.libretexts.org
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic Character Chemistry LibreTexts Are All Atoms Same Size Atoms of a given element are identical in size, mass, and other properties. Atoms cannot be subdivided, created, or destroyed. The fundamental characteristic that all atoms of the same. Each atom’s size is relative to the largest element, cesium. As you move down an element group (column), the size of atoms increases. This periodic table shows the relative sizes of. Are All Atoms Same Size.
From examples.yourdictionary.com
Difference Between Atoms and Elements (With Examples) Are All Atoms Same Size Atoms of different elements differ in size, mass, and other properties. The fundamental characteristic that all atoms of the same. As you move down an element group (column), the size of atoms increases. Atoms of a given element are identical in size, mass, and other properties. Each atom’s size is relative to the largest element, cesium. This periodic table shows. Are All Atoms Same Size.
From www.slideserve.com
PPT Atomic Size PowerPoint Presentation, free download ID6875591 Are All Atoms Same Size What makes atoms of different elements different? All atoms are made from the same bits, which are called subatomic particles (sub means smaller than and these are particles. This is because each atom further down the column has more protons and neutrons and also gains an. Atoms of different elements differ in size, mass, and other properties. The fundamental characteristic. Are All Atoms Same Size.