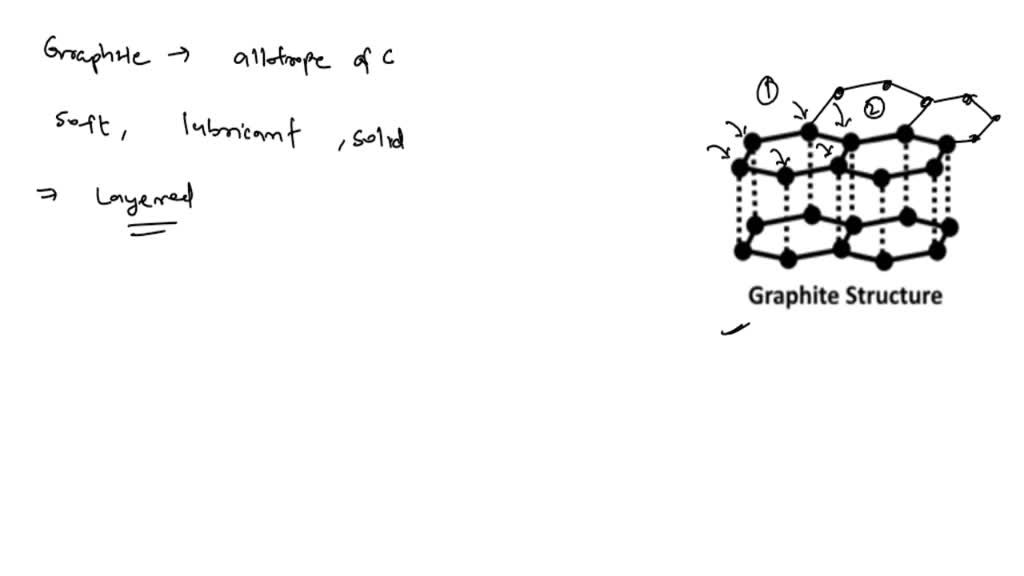
Why Is Graphite Used As A Lubricant Bbc Bitesize . In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able to slide over each other without interrupting the. It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. Scientists have recently developed a method to produce large sheets of a. Therefore, the layers of carbon atoms are able. Explain, in terms of its structure, why graphite can be used as a lubricant. It is a component of many lubricants, for example bicycle chain oil. Graphene is a substance made from carbon which consists of a single layer of graphite just one atom thick. Explain why diamond does not conduct electricity and why graphite does conduct electricity. Graphite is insoluble in water. Between the carbon layers in graphite there are van der waal forces which are very weak. Graphite is often used as a lubricant due to its slippery texture. It has a high melting point and is a good.
from www.numerade.com
It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. It is a component of many lubricants, for example bicycle chain oil. Therefore, the layers of carbon atoms are able. Graphite is insoluble in water. Scientists have recently developed a method to produce large sheets of a. Graphene is a substance made from carbon which consists of a single layer of graphite just one atom thick. In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able to slide over each other without interrupting the. Graphite is often used as a lubricant due to its slippery texture. Explain why diamond does not conduct electricity and why graphite does conduct electricity. Between the carbon layers in graphite there are van der waal forces which are very weak.
SOLVEDGraphite is a soft, solid, lubricant, extremely difficult to melt. The reason for this
Why Is Graphite Used As A Lubricant Bbc Bitesize It has a high melting point and is a good. Graphite is often used as a lubricant due to its slippery texture. Scientists have recently developed a method to produce large sheets of a. Explain, in terms of its structure, why graphite can be used as a lubricant. In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able to slide over each other without interrupting the. Therefore, the layers of carbon atoms are able. It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. It is a component of many lubricants, for example bicycle chain oil. Graphene is a substance made from carbon which consists of a single layer of graphite just one atom thick. It has a high melting point and is a good. Between the carbon layers in graphite there are van der waal forces which are very weak. Explain why diamond does not conduct electricity and why graphite does conduct electricity. Graphite is insoluble in water.
From www.quanswer.com
Why is graphite used as a lubricant while diamond is used as as an abrasive? Quanswer Why Is Graphite Used As A Lubricant Bbc Bitesize It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. Graphite is often used as a lubricant due to its slippery texture. In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able to slide over each other without interrupting the. Graphite is insoluble in water. Explain, in terms of. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.ehow.co.uk
Why is graphite a good lubricant? eHow UK Why Is Graphite Used As A Lubricant Bbc Bitesize Explain, in terms of its structure, why graphite can be used as a lubricant. It is a component of many lubricants, for example bicycle chain oil. In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able to slide over each other without interrupting the. Scientists have recently developed a method to produce large sheets of. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.slideserve.com
PPT Chapter 14 PowerPoint Presentation, free download ID1280057 Why Is Graphite Used As A Lubricant Bbc Bitesize Graphite is often used as a lubricant due to its slippery texture. Graphite is insoluble in water. It is a component of many lubricants, for example bicycle chain oil. It has a high melting point and is a good. Between the carbon layers in graphite there are van der waal forces which are very weak. Graphene is a substance made. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From blog.pencils.com
5 Unique Properties of Graphite You (Probably) Didn't Know Why Is Graphite Used As A Lubricant Bbc Bitesize In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able to slide over each other without interrupting the. Graphite is often used as a lubricant due to its slippery texture. Therefore, the layers of carbon atoms are able. It has a high melting point and is a good. Between the carbon layers in graphite there. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From periodictable.com
Graphite lubricant, a sample of the element Carbon in the Periodic Table Why Is Graphite Used As A Lubricant Bbc Bitesize Graphite is often used as a lubricant due to its slippery texture. Explain why diamond does not conduct electricity and why graphite does conduct electricity. In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able to slide over each other without interrupting the. Explain, in terms of its structure, why graphite can be used as. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.youtube.com
Is graphite a lubricant? YouTube Why Is Graphite Used As A Lubricant Bbc Bitesize It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. Between the carbon layers in graphite there are van der waal forces which are very weak. Explain why diamond does not conduct electricity and why graphite does conduct electricity. It is a component of many lubricants, for example bicycle chain oil. Graphite is insoluble. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From xenum.com
Solid lubricants 3 Graphite oil additive Xenum Power of Technology Why Is Graphite Used As A Lubricant Bbc Bitesize It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. Between the carbon layers in graphite there are van der waal forces which are very weak. Therefore, the layers of carbon atoms are able. It has a high melting point and is a good. Explain why diamond does not conduct electricity and why graphite. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.youtube.com
Graphite is used as a lubricant. Explain. YouTube Why Is Graphite Used As A Lubricant Bbc Bitesize Explain why diamond does not conduct electricity and why graphite does conduct electricity. Explain, in terms of its structure, why graphite can be used as a lubricant. Graphite is often used as a lubricant due to its slippery texture. It has a high melting point and is a good. Between the carbon layers in graphite there are van der waal. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.farmandfleet.com
Victor Powdered Graphite Lubricant 225002778 Blain's Farm & Fleet Why Is Graphite Used As A Lubricant Bbc Bitesize In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able to slide over each other without interrupting the. Graphite is insoluble in water. Between the carbon layers in graphite there are van der waal forces which are very weak. Explain, in terms of its structure, why graphite can be used as a lubricant. Scientists have. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.youtube.com
Class 11//why graphite is lubricant,why diamond isan abrasive explanation in Telugu YouTube Why Is Graphite Used As A Lubricant Bbc Bitesize Graphite is insoluble in water. Scientists have recently developed a method to produce large sheets of a. It has a high melting point and is a good. Explain why diamond does not conduct electricity and why graphite does conduct electricity. It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. Between the carbon layers. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.youtube.com
Graphite is a soft solid lubricant extremely difficult to melt. The reason for this anomalous Why Is Graphite Used As A Lubricant Bbc Bitesize In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able to slide over each other without interrupting the. Explain why diamond does not conduct electricity and why graphite does conduct electricity. Graphite is insoluble in water. It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. Between the carbon. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From soldered.com
Graphitebased lubricant 100 ml Why Is Graphite Used As A Lubricant Bbc Bitesize Graphene is a substance made from carbon which consists of a single layer of graphite just one atom thick. Explain why diamond does not conduct electricity and why graphite does conduct electricity. In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able to slide over each other without interrupting the. Scientists have recently developed a. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.bbc.co.uk
Photosynthesis BBC Bitesize Why Is Graphite Used As A Lubricant Bbc Bitesize It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. Graphene is a substance made from carbon which consists of a single layer of graphite just one atom thick. Therefore, the layers of carbon atoms are able. Scientists have recently developed a method to produce large sheets of a. Explain why diamond does not. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From hxetmqwny.blob.core.windows.net
Why Graphite Is Used As Lubricant Class 10 at Christopher Metz blog Why Is Graphite Used As A Lubricant Bbc Bitesize Therefore, the layers of carbon atoms are able. Between the carbon layers in graphite there are van der waal forces which are very weak. It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. It is a component of many lubricants, for example bicycle chain oil. Explain, in terms of its structure, why graphite. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From hxetmqwny.blob.core.windows.net
Why Graphite Is Used As Lubricant Class 10 at Christopher Metz blog Why Is Graphite Used As A Lubricant Bbc Bitesize It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able to slide over each other without interrupting the. It is a component of many lubricants, for example bicycle chain oil. Graphite is insoluble in water. Explain, in terms of. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.bbc.co.uk
Graphite Structures (CCEA) GCSE Chemistry (Single Science) Revision CCEA BBC Bitesize Why Is Graphite Used As A Lubricant Bbc Bitesize It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. Therefore, the layers of carbon atoms are able. Graphite is insoluble in water. Scientists have recently developed a method to produce large sheets of a. Explain why diamond does not conduct electricity and why graphite does conduct electricity. In graphite, the cations are arranged. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From whatispiping.com
Why Is Graphite a Better Lubricant Than Oil? Why Is Graphite Used As A Lubricant Bbc Bitesize Scientists have recently developed a method to produce large sheets of a. Explain, in terms of its structure, why graphite can be used as a lubricant. It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. It is a component of many lubricants, for example bicycle chain oil. Graphite is often used as a. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From bizfluent.com
Why Can Graphite Be Used As a Lubricant? Bizfluent Why Is Graphite Used As A Lubricant Bbc Bitesize Explain why diamond does not conduct electricity and why graphite does conduct electricity. Graphite is insoluble in water. Explain, in terms of its structure, why graphite can be used as a lubricant. Graphene is a substance made from carbon which consists of a single layer of graphite just one atom thick. Scientists have recently developed a method to produce large. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.tassoalloys.com
Scientific fundamentals Graphite Usage As A Lubricant Why Is Graphite Used As A Lubricant Bbc Bitesize In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able to slide over each other without interrupting the. Scientists have recently developed a method to produce large sheets of a. Explain, in terms of its structure, why graphite can be used as a lubricant. Between the carbon layers in graphite there are van der waal. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From rainbowtech.net
Dry Graphite Lubricant Rainbow Technology Why Is Graphite Used As A Lubricant Bbc Bitesize Graphene is a substance made from carbon which consists of a single layer of graphite just one atom thick. It is a component of many lubricants, for example bicycle chain oil. Explain, in terms of its structure, why graphite can be used as a lubricant. Graphite is insoluble in water. Between the carbon layers in graphite there are van der. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.dgfspray.com
Graphite as lubricant — Miracle Power Products Why Is Graphite Used As A Lubricant Bbc Bitesize Therefore, the layers of carbon atoms are able. Explain, in terms of its structure, why graphite can be used as a lubricant. Between the carbon layers in graphite there are van der waal forces which are very weak. Graphite is insoluble in water. It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. Graphite. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From hxeoizqfs.blob.core.windows.net
Graphite Is Used As A Lubricant In Machines Because at Bernadine Parr blog Why Is Graphite Used As A Lubricant Bbc Bitesize Explain why diamond does not conduct electricity and why graphite does conduct electricity. Scientists have recently developed a method to produce large sheets of a. Graphite is often used as a lubricant due to its slippery texture. Between the carbon layers in graphite there are van der waal forces which are very weak. Graphene is a substance made from carbon. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.ftmmachinery.com
9 Interesting Questions About Graphite Uses Fote Machinery Why Is Graphite Used As A Lubricant Bbc Bitesize Scientists have recently developed a method to produce large sheets of a. Explain, in terms of its structure, why graphite can be used as a lubricant. Therefore, the layers of carbon atoms are able. Graphene is a substance made from carbon which consists of a single layer of graphite just one atom thick. Graphite is insoluble in water. It is. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From byjus.com
Give reason Graphite is used as a lubricant? Why Is Graphite Used As A Lubricant Bbc Bitesize Between the carbon layers in graphite there are van der waal forces which are very weak. Therefore, the layers of carbon atoms are able. Explain why diamond does not conduct electricity and why graphite does conduct electricity. Explain, in terms of its structure, why graphite can be used as a lubricant. Scientists have recently developed a method to produce large. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From substech.com
Graphite as solid lubricant [SubsTech] Why Is Graphite Used As A Lubricant Bbc Bitesize Explain, in terms of its structure, why graphite can be used as a lubricant. Graphite is often used as a lubricant due to its slippery texture. Graphite is insoluble in water. Therefore, the layers of carbon atoms are able. It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. Graphene is a substance made. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.alamy.com
Graphite. Computer graphics representation of the structure of graphite. Graphite, used in Why Is Graphite Used As A Lubricant Bbc Bitesize Graphene is a substance made from carbon which consists of a single layer of graphite just one atom thick. Therefore, the layers of carbon atoms are able. It has a high melting point and is a good. Between the carbon layers in graphite there are van der waal forces which are very weak. It is also used as the “lead”. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.homedepot.ca
B'laster Graphite Dry Lubricant 156g The Home Depot Canada Why Is Graphite Used As A Lubricant Bbc Bitesize Graphene is a substance made from carbon which consists of a single layer of graphite just one atom thick. It is also used as the “lead” in pencils, as a moderator in nuclear reactors, and. It is a component of many lubricants, for example bicycle chain oil. Between the carbon layers in graphite there are van der waal forces which. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.doubtnut.com
[Telugu] Why graphite can be used as a lubricant? Why Is Graphite Used As A Lubricant Bbc Bitesize In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able to slide over each other without interrupting the. Between the carbon layers in graphite there are van der waal forces which are very weak. Graphite is insoluble in water. It has a high melting point and is a good. It is also used as the. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.numerade.com
SOLVEDGraphite is a soft, solid, lubricant, extremely difficult to melt. The reason for this Why Is Graphite Used As A Lubricant Bbc Bitesize Therefore, the layers of carbon atoms are able. Graphite is insoluble in water. Explain why diamond does not conduct electricity and why graphite does conduct electricity. Graphene is a substance made from carbon which consists of a single layer of graphite just one atom thick. In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From abro.com
Graphite Powder Lubricant ABRO Why Is Graphite Used As A Lubricant Bbc Bitesize It is a component of many lubricants, for example bicycle chain oil. Graphite is often used as a lubricant due to its slippery texture. In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able to slide over each other without interrupting the. Between the carbon layers in graphite there are van der waal forces which. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.dreamstime.com
Graphite Powder is Generally Used As Lubricant Stock Photo Image of isolated, closeup 208083130 Why Is Graphite Used As A Lubricant Bbc Bitesize It is a component of many lubricants, for example bicycle chain oil. In graphite, the cations are arranged in layers (sheets of carbon atoms)the layers are able to slide over each other without interrupting the. Graphene is a substance made from carbon which consists of a single layer of graphite just one atom thick. Graphite is insoluble in water. Between. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.bbc.co.uk
Charcoal and graphite BBC Bitesize Why Is Graphite Used As A Lubricant Bbc Bitesize Explain, in terms of its structure, why graphite can be used as a lubricant. Therefore, the layers of carbon atoms are able. Scientists have recently developed a method to produce large sheets of a. Explain why diamond does not conduct electricity and why graphite does conduct electricity. It is also used as the “lead” in pencils, as a moderator in. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.savemyexams.com
Diamond, Graphite & Silicon (IV) Oxide Cambridge O Level Chemistry Revision Notes 2023 Why Is Graphite Used As A Lubricant Bbc Bitesize Graphene is a substance made from carbon which consists of a single layer of graphite just one atom thick. It is a component of many lubricants, for example bicycle chain oil. Graphite is often used as a lubricant due to its slippery texture. Therefore, the layers of carbon atoms are able. In graphite, the cations are arranged in layers (sheets. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From hxetmqwny.blob.core.windows.net
Why Graphite Is Used As Lubricant Class 10 at Christopher Metz blog Why Is Graphite Used As A Lubricant Bbc Bitesize Explain why diamond does not conduct electricity and why graphite does conduct electricity. Scientists have recently developed a method to produce large sheets of a. Therefore, the layers of carbon atoms are able. Between the carbon layers in graphite there are van der waal forces which are very weak. It has a high melting point and is a good. In. Why Is Graphite Used As A Lubricant Bbc Bitesize.
From www.youtube.com
How do Solid Lubricants like MoS2, Graphite and hBN work? YouTube Why Is Graphite Used As A Lubricant Bbc Bitesize Graphite is often used as a lubricant due to its slippery texture. It has a high melting point and is a good. Explain why diamond does not conduct electricity and why graphite does conduct electricity. Graphite is insoluble in water. Scientists have recently developed a method to produce large sheets of a. It is also used as the “lead” in. Why Is Graphite Used As A Lubricant Bbc Bitesize.