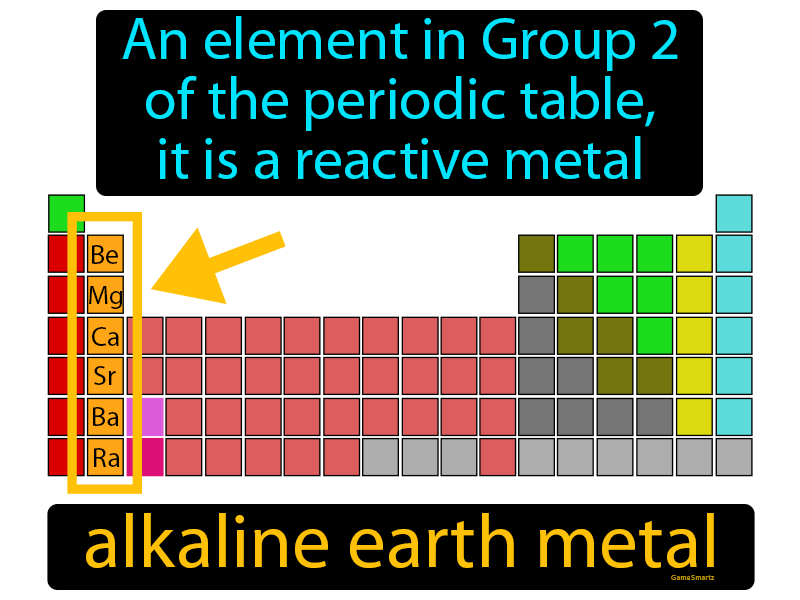
Basic Properties Of Alkaline Earth Metals . Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and ‘basic’ or ‘alkaline’ properties [5]. Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. The elements are beryllium (be), magnesium (mg), calcium (ca),. They are beryllium (be), magnesium (mg), calcium (ca),. The alkaline earth metals tend to form +2. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. The alkaline earth metals are six chemical elements in group 2 of the periodic table.
from cabinet.matttroy.net
Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. The elements are beryllium (be), magnesium (mg), calcium (ca),. Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. They are beryllium (be), magnesium (mg), calcium (ca),. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and ‘basic’ or ‘alkaline’ properties [5]. The alkaline earth metals are six chemical elements in group 2 of the periodic table. The alkaline earth metals tend to form +2. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state.
Alkaline Earth Metals Periodic Table Definition Matttroy
Basic Properties Of Alkaline Earth Metals The alkaline earth metals tend to form +2. The alkaline earth metals tend to form +2. Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. The elements are beryllium (be), magnesium (mg), calcium (ca),. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. The alkaline earth metals are six chemical elements in group 2 of the periodic table. The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and ‘basic’ or ‘alkaline’ properties [5]. They are beryllium (be), magnesium (mg), calcium (ca),. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom.
From www.youtube.com
Chemical properties of alkaline earth metals / Alkali & alkaline earth Basic Properties Of Alkaline Earth Metals Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. The alkaline earth metals are six chemical elements in group 2 of the periodic table. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. They are beryllium (be), magnesium (mg), calcium (ca),. The alkaline earth metals tend to form +2. Alkaline earth metals dissolve in. Basic Properties Of Alkaline Earth Metals.
From www.animalia-life.club
Periodic Table Of Elements Alkaline Earth Metals Basic Properties Of Alkaline Earth Metals Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (be), magnesium (mg), calcium (ca),. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. The alkaline earth metals tend to. Basic Properties Of Alkaline Earth Metals.
From www.slideserve.com
PPT Elements and their Properties PowerPoint Presentation, free Basic Properties Of Alkaline Earth Metals Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. The alkaline metals are named as such because when mixed with. Basic Properties Of Alkaline Earth Metals.
From byjus.com
Alkaline Earth Metals Occurrence and Extraction,Physical Properties Basic Properties Of Alkaline Earth Metals The elements are beryllium (be), magnesium (mg), calcium (ca),. Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and ‘basic’ or ‘alkaline’ properties [5]. They are. Basic Properties Of Alkaline Earth Metals.
From studylib.net
Alkaline Earth Metals Basic Properties Of Alkaline Earth Metals The alkaline earth metals are six chemical elements in group 2 of the periodic table. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. They are beryllium (be), magnesium (mg), calcium (ca),. The elements are beryllium (be), magnesium (mg), calcium (ca),. The alkaline earth metals tend to form +2. Alkaline earth metals dissolve. Basic Properties Of Alkaline Earth Metals.
From www.slideserve.com
PPT ELEMENT CLASSES PowerPoint Presentation ID149914 Basic Properties Of Alkaline Earth Metals The alkaline earth metals tend to form +2. They are beryllium (be), magnesium (mg), calcium (ca),. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. The elements are beryllium (be), magnesium (mg), calcium (ca),. Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. Alkaline earth metals dissolve in liquid ammonia to give solutions that. Basic Properties Of Alkaline Earth Metals.
From www.youtube.com
18. Properties Of Alkaline Earth Metals YouTube Basic Properties Of Alkaline Earth Metals Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. The alkaline earth metals are six chemical elements in group 2 of the periodic table. The alkaline earth metals tend to form +2. The alkaline metals. Basic Properties Of Alkaline Earth Metals.
From www.slideserve.com
PPT Alkaline Earth Metals PowerPoint Presentation, free download ID Basic Properties Of Alkaline Earth Metals Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. The elements are beryllium (be), magnesium (mg), calcium (ca),. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher. Basic Properties Of Alkaline Earth Metals.
From www.slideserve.com
PPT Alkaline Earth Metals PowerPoint Presentation ID2046855 Basic Properties Of Alkaline Earth Metals The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and ‘basic’ or ‘alkaline’ properties [5]. The alkaline earth metals are six chemical elements in group 2 of the periodic table. Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. Alkaline earth metals dissolve in liquid ammonia to give solutions that. Basic Properties Of Alkaline Earth Metals.
From cabinet.matttroy.net
Alkaline Earth Metals Periodic Table Definition Matttroy Basic Properties Of Alkaline Earth Metals They are beryllium (be), magnesium (mg), calcium (ca),. The elements are beryllium (be), magnesium (mg), calcium (ca),. Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7,. Basic Properties Of Alkaline Earth Metals.
From www.vrogue.co
Family Of Alkali Metals Alkaline Earth Metals Alkalin vrogue.co Basic Properties Of Alkaline Earth Metals Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and ‘basic’ or ‘alkaline’ properties [5]. The. Basic Properties Of Alkaline Earth Metals.
From www.sliderbase.com
Element Classes Presentation Chemistry Basic Properties Of Alkaline Earth Metals Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. The alkaline earth metals tend to form +2. The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and ‘basic’ or ‘alkaline’ properties [5]. The alkaline earth metals are six chemical elements in group. Basic Properties Of Alkaline Earth Metals.
From fransiscafransiscanoraleser0253503.blogspot.com
Venn Diagram Of Metals Nonmetals And Metalloids / The Difference Basic Properties Of Alkaline Earth Metals The alkaline earth metals tend to form +2. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. The alkaline earth metals are six chemical elements in group 2 of the periodic table. The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and ‘basic’. Basic Properties Of Alkaline Earth Metals.
From examples.yourdictionary.com
Basic Types of Metals on the Periodic Table Basic Properties Of Alkaline Earth Metals Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. The elements are beryllium (be), magnesium (mg), calcium (ca),. The alkaline earth metals are six chemical elements in group 2 of the periodic table. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. The alkaline metals are named as such because when mixed. Basic Properties Of Alkaline Earth Metals.
From www.youtube.com
Physical properties of alkaline earth metals SBlock elements bsc Basic Properties Of Alkaline Earth Metals Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. They are beryllium (be), magnesium (mg), calcium (ca),. The elements are beryllium (be), magnesium (mg), calcium (ca),. Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. The alkaline earth metals tend to form +2. The alkaline earth metals are six chemical elements in group 2. Basic Properties Of Alkaline Earth Metals.
From scienceinfo.com
Comparison of properties of Alkali and Alkaline Earth Metals Basic Properties Of Alkaline Earth Metals They are beryllium (be), magnesium (mg), calcium (ca),. Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. Chemical properties of alkali earth metals covers the elements beryllium (be),. Basic Properties Of Alkaline Earth Metals.
From www.britannica.com
Alkalineearth metal chemical element Britannica Basic Properties Of Alkaline Earth Metals The alkaline earth metals are six chemical elements in group 2 of the periodic table. Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. The elements are beryllium (be), magnesium (mg), calcium (ca),. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Chemical properties of alkali earth metals covers the elements beryllium. Basic Properties Of Alkaline Earth Metals.
From overallscience.com
Trends in atomic and physical properties of alkali metals Overall Science Basic Properties Of Alkaline Earth Metals The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and ‘basic’ or ‘alkaline’ properties [5]. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. The elements are beryllium (be), magnesium (mg), calcium (ca),. Alkaline earth. Basic Properties Of Alkaline Earth Metals.
From www.youtube.com
Chemical properties of Alkali metals / Part 3 / Alkali & alkaline Basic Properties Of Alkaline Earth Metals The alkaline earth metals are six chemical elements in group 2 of the periodic table. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. The alkaline earth metals tend to form +2. The elements are. Basic Properties Of Alkaline Earth Metals.
From www.purposegames.com
Name the Alkaline Earth Metals Quiz Basic Properties Of Alkaline Earth Metals They are beryllium (be), magnesium (mg), calcium (ca),. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. The alkaline earth metals are six chemical elements in group 2 of the periodic table. The alkaline earth metals tend to. Basic Properties Of Alkaline Earth Metals.
From www.youtube.com
Occurrence of ALKALI & ALKALINE Earth Metals SBlock Elements F Basic Properties Of Alkaline Earth Metals The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and ‘basic’ or ‘alkaline’ properties [5]. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. The alkaline earth metals are six chemical elements in group 2 of the periodic table. Alkaline earth metals. Basic Properties Of Alkaline Earth Metals.
From byjus.com
Alkaline Earth Metals General Characteristics of Oxides, Hallides Basic Properties Of Alkaline Earth Metals Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and ‘basic’ or ‘alkaline’ properties [5]. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. The elements are. Basic Properties Of Alkaline Earth Metals.
From www.online-sciences.com
Elements of sblock, Properties of the first group elements 1A (Alkali Basic Properties Of Alkaline Earth Metals Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. The alkaline earth metals tend to form +2. The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and ‘basic’ or ‘alkaline’ properties [5]. The alkaline earth metals are six chemical elements. Basic Properties Of Alkaline Earth Metals.
From www.nagwa.com
Question Video Identifying Alkaline Earth Metals Nagwa Basic Properties Of Alkaline Earth Metals The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and ‘basic’ or ‘alkaline’ properties [5]. They are beryllium (be), magnesium (mg), calcium (ca),. The elements are beryllium (be), magnesium (mg), calcium (ca),. Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. Chemical properties of alkali earth metals covers the elements. Basic Properties Of Alkaline Earth Metals.
From assign.unaux.com
Periodic properties of alkali metals and halogens. assign Basic Properties Of Alkaline Earth Metals Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. The alkaline earth metals tend to form +2. The elements are beryllium (be), magnesium (mg), calcium (ca),. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. The alkaline metals are. Basic Properties Of Alkaline Earth Metals.
From www.expii.com
Alkaline Earth Metals — Overview & Properties Expii Basic Properties Of Alkaline Earth Metals The alkaline earth metals tend to form +2. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and. Basic Properties Of Alkaline Earth Metals.
From www.slideserve.com
PPT METALS PowerPoint Presentation, free download ID3352504 Basic Properties Of Alkaline Earth Metals The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and ‘basic’ or ‘alkaline’ properties [5]. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. The alkaline earth. Basic Properties Of Alkaline Earth Metals.
From newtondesk.com
Alkaline Earth Metals On The Periodic Table Chemistry Elements Basic Properties Of Alkaline Earth Metals Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. The alkaline earth metals tend to form +2. The alkaline earth metals are six chemical elements in group 2 of the periodic table. The elements are beryllium (be), magnesium (mg), calcium (ca),. Alkaline earth metals are. Basic Properties Of Alkaline Earth Metals.
From www.youtube.com
Properties of Alkali and Alkaline Earth Metals Hydroxides, Chemistry Basic Properties Of Alkaline Earth Metals The elements are beryllium (be), magnesium (mg), calcium (ca),. Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. The alkaline earth metals tend to form +2. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. They. Basic Properties Of Alkaline Earth Metals.
From ar.inspiredpencil.com
Alkaline Earth Metals Pictures Basic Properties Of Alkaline Earth Metals The elements are beryllium (be), magnesium (mg), calcium (ca),. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. They are beryllium (be), magnesium (mg), calcium (ca),. The alkaline metals are named as such because when mixed. Basic Properties Of Alkaline Earth Metals.
From utedzz.blogspot.com
Periodic Table Showing Alkali Metals Alkaline Earth Metals Periodic Basic Properties Of Alkaline Earth Metals Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. The elements are beryllium (be), magnesium (mg), calcium (ca),. The alkaline earth metals are six chemical elements in group 2 of the periodic table. The alkaline earth metals tend. Basic Properties Of Alkaline Earth Metals.
From ar.inspiredpencil.com
Periodic Table Of Elements Alkaline Earth Metals Basic Properties Of Alkaline Earth Metals Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. The alkaline earth metals are six chemical elements in group 2 of the periodic table. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. The elements are beryllium (be), magnesium (mg), calcium (ca),. The alkaline earth metals tend. Basic Properties Of Alkaline Earth Metals.
From www.scribd.com
Trends in Group 2 Elements An Analysis of the Properties of Alkaline Basic Properties Of Alkaline Earth Metals The elements are beryllium (be), magnesium (mg), calcium (ca),. Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state. Chemical properties of alkali earth metals covers the elements beryllium (be), magnesium (mg), calcium (ca), strontium. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. The alkaline. Basic Properties Of Alkaline Earth Metals.
From www.slideserve.com
PPT ELEMENTS CHEMICAL & PHYSICAL PROPERTIES PowerPoint Presentation Basic Properties Of Alkaline Earth Metals They are beryllium (be), magnesium (mg), calcium (ca),. The alkaline metals are named as such because when mixed with water, they produce solutions with ph higher than 7, and ‘basic’ or ‘alkaline’ properties [5]. Understand properties, electronic configuration, analogous behaviour, reactivity, ionization. The alkaline earth metals are six chemical elements in group 2 of the periodic table. The alkaline earth. Basic Properties Of Alkaline Earth Metals.
From www.youtube.com
Properties of Alkali and Alkaline Earth Metals Oxides, Chemistry Basic Properties Of Alkaline Earth Metals The alkaline earth metals are six chemical elements in group 2 of the periodic table. The elements are beryllium (be), magnesium (mg), calcium (ca),. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. The alkaline earth metals tend to form +2. They are beryllium (be), magnesium (mg), calcium (ca),. Alkaline earth. Basic Properties Of Alkaline Earth Metals.