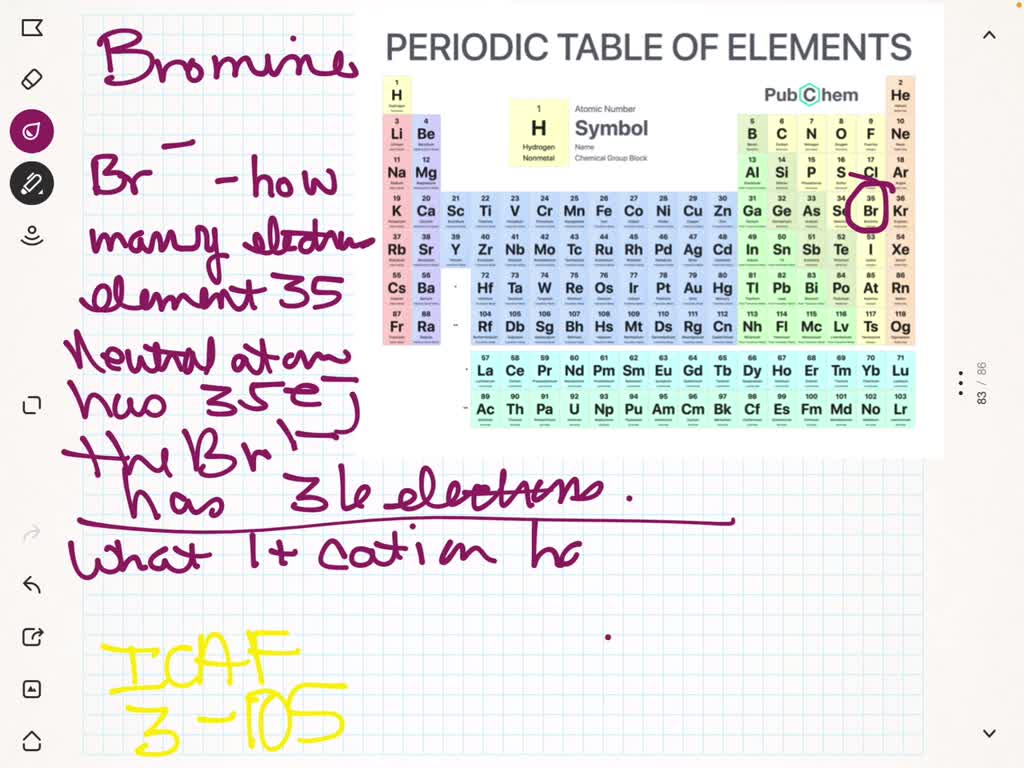
Bromine Number Of Electrons Gained Or Lost . Electron configurations can be used to show how many. An ion is a charged particle formed when an atom or molecule loses or gains electrons from the outer shells, causing the number of protons and electrons to become unequal. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Complete the chart using your knowledge of atoms.
from www.numerade.com
Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Electron configurations can be used to show how many. An ion is a charged particle formed when an atom or molecule loses or gains electrons from the outer shells, causing the number of protons and electrons to become unequal. Complete the chart using your knowledge of atoms.
SOLVEDA bromide ion has gained one electron. It is symbolized by Br
Bromine Number Of Electrons Gained Or Lost You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Complete the chart using your knowledge of atoms. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. Electron configurations can be used to show how many. An ion is a charged particle formed when an atom or molecule loses or gains electrons from the outer shells, causing the number of protons and electrons to become unequal. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Number Of Electrons Gained Or Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. Electron configurations can be used to show how many. An ion is a charged particle formed when an atom or. Bromine Number Of Electrons Gained Or Lost.
From learningfullsharpen.z21.web.core.windows.net
How To Determine A Valence Electron Bromine Number Of Electrons Gained Or Lost A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). Complete the chart using your knowledge of. Bromine Number Of Electrons Gained Or Lost.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Bromine Number Of Electrons Gained Or Lost Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). An ion is a charged particle formed when an atom or molecule loses or gains electrons from the outer shells, causing the number of protons and electrons to become unequal. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Bromine Number Of Electrons Gained Or Lost.
From www.numerade.com
SOLVEDA bromide ion has gained one electron. It is symbolized by Br Bromine Number Of Electrons Gained Or Lost Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). Complete the chart using your knowledge of atoms. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. You may assume the valences of the chemical. Bromine Number Of Electrons Gained Or Lost.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Bromine Number Of Electrons Gained Or Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Atoms tend to gain or lose electrons to. Bromine Number Of Electrons Gained Or Lost.
From favpng.com
Electron Configuration Bromine Chemical Element Electron Shell Bohr Bromine Number Of Electrons Gained Or Lost You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. Complete the chart using your knowledge of atoms. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. A positive or negative integer that represents the number. Bromine Number Of Electrons Gained Or Lost.
From www.youtube.com
Determining the number of electrons lost or gained YouTube Bromine Number Of Electrons Gained Or Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). Electron. Bromine Number Of Electrons Gained Or Lost.
From answerfullallan101.z19.web.core.windows.net
Losing And Gaining Electrons Worksheet Bromine Number Of Electrons Gained Or Lost Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. An ion is a charged particle formed when an atom or molecule loses or gains electrons from the outer shells, causing the number. Bromine Number Of Electrons Gained Or Lost.
From www.alamy.com
Symbol and electron diagram for Bromine illustration Stock Vector Image Bromine Number Of Electrons Gained Or Lost A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. An ion is a charged particle formed when an atom or molecule loses or gains electrons from the outer shells, causing the number of protons and electrons to become unequal. Electron configurations can be used to show how. Bromine Number Of Electrons Gained Or Lost.
From www.youtube.com
How to Find the Valence Electrons for Bromine (Br) YouTube Bromine Number Of Electrons Gained Or Lost Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Complete the chart using your knowledge of atoms. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Electron configurations can be used to show how many. A positive or negative integer that represents the number of electrons that. Bromine Number Of Electrons Gained Or Lost.
From www.chegg.com
Solved Atoms Forming Ions Directions Write out equations Bromine Number Of Electrons Gained Or Lost A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. You may assume the valences of the chemical elements—the number of electrons with which an. Bromine Number Of Electrons Gained Or Lost.
From www.shutterstock.com
Quantum Numbers Over 3,097 RoyaltyFree Licensable Stock Vectors Bromine Number Of Electrons Gained Or Lost Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. Complete the chart using your knowledge of atoms. Electron configurations can be used to show how. Bromine Number Of Electrons Gained Or Lost.
From www.youtube.com
How many valence electrons does bromine have? YouTube Bromine Number Of Electrons Gained Or Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. A positive or negative integer that represents the number of electrons that. Bromine Number Of Electrons Gained Or Lost.
From hxebeffzi.blob.core.windows.net
Bromine Mass Of Atom at Margaret Duppstadt blog Bromine Number Of Electrons Gained Or Lost A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. An ion is. Bromine Number Of Electrons Gained Or Lost.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Number Of Electrons Gained Or Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a. Bromine Number Of Electrons Gained Or Lost.
From www.wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Bromine Number Of Electrons Gained Or Lost An ion is a charged particle formed when an atom or molecule loses or gains electrons from the outer shells, causing the number of protons and electrons to become unequal. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. The atomic number for bromine is 35, which means. Bromine Number Of Electrons Gained Or Lost.
From quizdbpharmacies.z4.web.core.windows.net
Number Of Protons And Neutrons In Fluorine Bromine Number Of Electrons Gained Or Lost A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Complete the chart using your knowledge of atoms. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. Atoms tend to lose, gain,. Bromine Number Of Electrons Gained Or Lost.
From www.collegesidekick.com
Lewis Symbols and Structures Chemistry I Bromine Number Of Electrons Gained Or Lost Electron configurations can be used to show how many. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). The atomic number for bromine is. Bromine Number Of Electrons Gained Or Lost.
From carisca.github.io
Electron Configuration Of Cr+ Valence Lewis Structure Draw Electrons Bromine Number Of Electrons Gained Or Lost An ion is a charged particle formed when an atom or molecule loses or gains electrons from the outer shells, causing the number of protons and electrons to become unequal. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. You may assume the valences of the chemical elements—the number of electrons with. Bromine Number Of Electrons Gained Or Lost.
From valenceelectrons.com
Silver(Ag) electron configuration and orbital diagram Bromine Number Of Electrons Gained Or Lost Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Complete the chart using your knowledge of atoms. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. A positive or negative. Bromine Number Of Electrons Gained Or Lost.
From exocnoogo.blob.core.windows.net
Chlorine Number Of Electrons Gained Or Lost at Iva Swearingen blog Bromine Number Of Electrons Gained Or Lost An ion is a charged particle formed when an atom or molecule loses or gains electrons from the outer shells, causing the number of protons and electrons to become unequal. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. The atomic number for bromine is 35, which. Bromine Number Of Electrons Gained Or Lost.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Number Of Electrons Gained Or Lost Electron configurations can be used to show how many. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). Complete the chart using your knowledge of atoms. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. An ion is a charged particle formed. Bromine Number Of Electrons Gained Or Lost.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Bromine Number Of Electrons Gained Or Lost Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. Electron configurations can be used to show how many. Atoms tend to lose, gain,. Bromine Number Of Electrons Gained Or Lost.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine Number Of Electrons Gained Or Lost Complete the chart using your knowledge of atoms. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. You may assume the valences of the chemical elements—the number of electrons. Bromine Number Of Electrons Gained Or Lost.
From www.sciencecoverage.com
How Many Valence Electrons Does Bromine (Br) Have? [Valency of Bromine] Bromine Number Of Electrons Gained Or Lost Electron configurations can be used to show how many. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. An ion is a charged particle. Bromine Number Of Electrons Gained Or Lost.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Number Of Electrons Gained Or Lost Electron configurations can be used to show how many. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). An ion is a charged particle formed when an atom or molecule loses or gains electrons from the outer shells, causing the number of protons and electrons to become. Bromine Number Of Electrons Gained Or Lost.
From www.vrogue.co
Calculate The Number Of Electrons Lost Or Gained Duri vrogue.co Bromine Number Of Electrons Gained Or Lost An ion is a charged particle formed when an atom or molecule loses or gains electrons from the outer shells, causing the number of protons and electrons to become unequal. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. A. Bromine Number Of Electrons Gained Or Lost.
From www.slideserve.com
PPT 3 rd Orbitals PowerPoint Presentation, free download ID2062267 Bromine Number Of Electrons Gained Or Lost Complete the chart using your knowledge of atoms. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. An ion is a charged particle formed when an atom or molecule loses or gains electrons from the outer shells, causing the number of protons and electrons to become unequal. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons).. Bromine Number Of Electrons Gained Or Lost.
From app.emaze.com
Bromine ) on emaze Bromine Number Of Electrons Gained Or Lost You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. An ion is a charged particle formed when an atom or molecule loses or gains electrons from the outer shells, causing the. Bromine Number Of Electrons Gained Or Lost.
From www.youtube.com
How to find Protons & Electrons for the Bromide ion (Br) YouTube Bromine Number Of Electrons Gained Or Lost Complete the chart using your knowledge of atoms. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). A positive or negative integer that represents the number of electrons that an atom has gained or. Bromine Number Of Electrons Gained Or Lost.
From www.alamy.com
Protons And Neutrons Stock Photos & Protons And Neutrons Stock Images Bromine Number Of Electrons Gained Or Lost Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). Complete the chart using your knowledge of atoms. Electron configurations can be used to show how many. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those.. Bromine Number Of Electrons Gained Or Lost.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Number Of Electrons Gained Or Lost Complete the chart using your knowledge of atoms. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. Electron configurations can be used to show how many.. Bromine Number Of Electrons Gained Or Lost.
From oneclass.com
OneClass Write a possible full set of quantum numbers for the electron Bromine Number Of Electrons Gained Or Lost Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. Complete the chart using your knowledge of atoms. Electron configurations can be. Bromine Number Of Electrons Gained Or Lost.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration Bromine Number Of Electrons Gained Or Lost The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. Complete the chart using your knowledge of atoms. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. An ion is a charged particle formed when an atom or molecule loses or. Bromine Number Of Electrons Gained Or Lost.
From hxefrvyvj.blob.core.windows.net
Bromine Periodic Table Protons Electrons at Margaret Saenz blog Bromine Number Of Electrons Gained Or Lost Atoms tend to gain or lose electrons to achieve an octet (8 valence electrons). Electron configurations can be used to show how many. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Bromine Number Of Electrons Gained Or Lost.