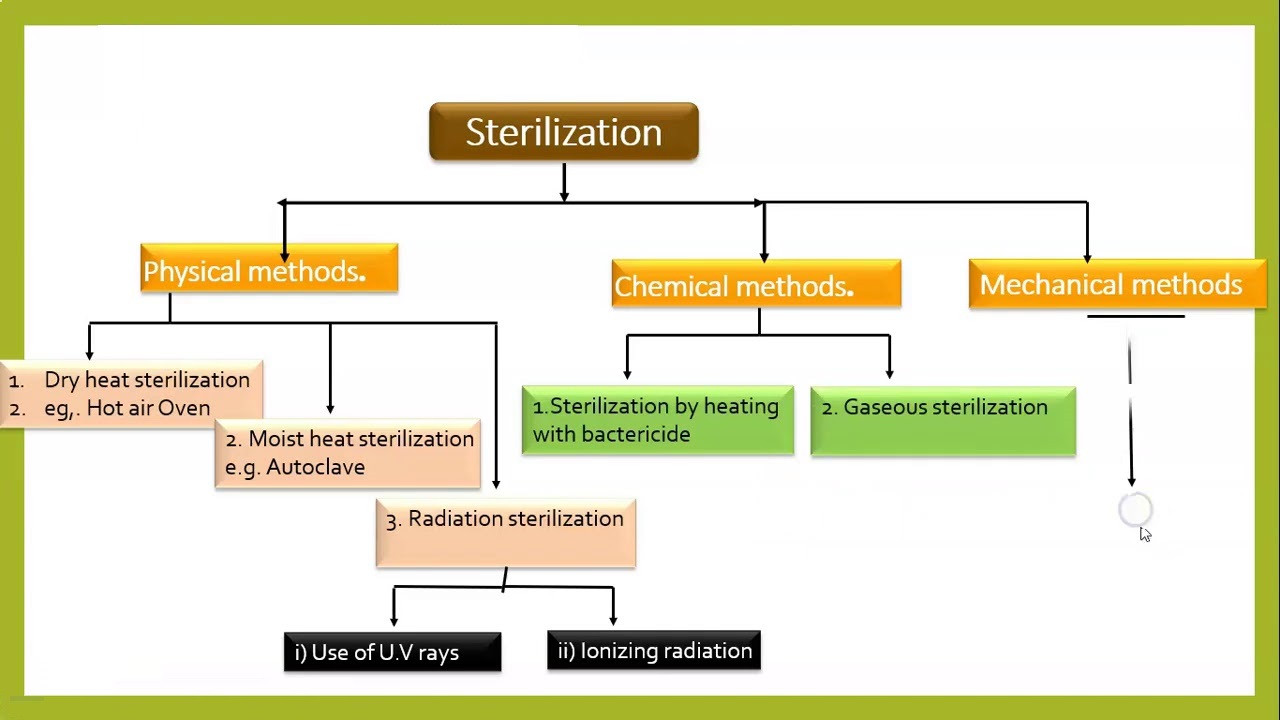
Sterile Filter Validation . There are eight elements to filter validation: Validation by sartorius ensures reliable production processes that. The chapter then covers how to develop, implement, and validate a. Complete your process validation with confidence ®. Sterile filters, their properties, manufacture, retention mechanisms, and economics are described. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. Focus on these 3 elements of validation sterile filter master extractables/ plan prove the stream does not adversely impact the filter. “the integrity of the sterilised filter should be verified before use and should be confirmed immediately after use by an appropriate. The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove.
from kerone.com
There are eight elements to filter validation: The chapter then covers how to develop, implement, and validate a. Sterile filters, their properties, manufacture, retention mechanisms, and economics are described. The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. “the integrity of the sterilised filter should be verified before use and should be confirmed immediately after use by an appropriate. Focus on these 3 elements of validation sterile filter master extractables/ plan prove the stream does not adversely impact the filter. Validation by sartorius ensures reliable production processes that. Complete your process validation with confidence ®.
sterilization
Sterile Filter Validation “the integrity of the sterilised filter should be verified before use and should be confirmed immediately after use by an appropriate. There are eight elements to filter validation: Complete your process validation with confidence ®. Validation by sartorius ensures reliable production processes that. Focus on these 3 elements of validation sterile filter master extractables/ plan prove the stream does not adversely impact the filter. “the integrity of the sterilised filter should be verified before use and should be confirmed immediately after use by an appropriate. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. Sterile filters, their properties, manufacture, retention mechanisms, and economics are described. The chapter then covers how to develop, implement, and validate a.
From prisystems.com
Sterile Filter Integrity Testing OnSite Factory Trained PRI Bio Sterile Filter Validation Validation by sartorius ensures reliable production processes that. Complete your process validation with confidence ®. The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. The chapter then covers how to develop, implement, and validate a. Focus on these 3 elements of validation sterile filter master extractables/ plan prove the stream does. Sterile Filter Validation.
From www.criticalprocess.com
Sterile Filtration and Bioburden Control for the Processing of Biologics Sterile Filter Validation Validation by sartorius ensures reliable production processes that. The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. “the integrity of the sterilised filter should be verified before use and should. Sterile Filter Validation.
From info.mesalabs.com
Pharmaceutical Aseptic Manufacturing Sterilization Validation Mesa Labs Sterile Filter Validation Sterile filters, their properties, manufacture, retention mechanisms, and economics are described. Validation by sartorius ensures reliable production processes that. Focus on these 3 elements of validation sterile filter master extractables/ plan prove the stream does not adversely impact the filter. There are eight elements to filter validation: Complete your process validation with confidence ®. The validation process involves testing and. Sterile Filter Validation.
From lubrizolcdmo.com
Phase Appropriate Filter Validation for Sterile Drug Product Sterile Filter Validation Complete your process validation with confidence ®. Focus on these 3 elements of validation sterile filter master extractables/ plan prove the stream does not adversely impact the filter. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. Validation by sartorius ensures reliable production processes that. Sterile filters, their. Sterile Filter Validation.
From microdyneproducts.com
Sterile Filters Set the Standard for Sterile Compressed Air Microdyne Sterile Filter Validation “the integrity of the sterilised filter should be verified before use and should be confirmed immediately after use by an appropriate. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. There are eight elements to filter validation: Sterile filters, their properties, manufacture, retention mechanisms, and economics are described.. Sterile Filter Validation.
From www.eurofins.it
filter validation for sterile drug manufacturing Eurofins Italia Sterile Filter Validation Sterile filters, their properties, manufacture, retention mechanisms, and economics are described. The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. The chapter then covers how to develop, implement, and validate a. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or. Sterile Filter Validation.
From www.sartorius.hr
Sartolon Sterile Filters Downstream Sartorius Croatia Sterile Filter Validation Validation by sartorius ensures reliable production processes that. Complete your process validation with confidence ®. The chapter then covers how to develop, implement, and validate a. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. There are eight elements to filter validation: The validation process involves testing and. Sterile Filter Validation.
From www.researchandmarkets.com
Sterile Filtration of Pharmaceutical Products Validation and Sterile Filter Validation The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. “the integrity of the sterilised filter should be verified before use and should be confirmed immediately after use by an appropriate. The chapter then covers how to develop, implement, and validate a. Sterile filters, their properties, manufacture, retention mechanisms, and economics are. Sterile Filter Validation.
From www.learngxp.com
Considerations of Sterile Filtration Validation 21 CFR 211.113 (b Sterile Filter Validation There are eight elements to filter validation: Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. Validation by sartorius ensures reliable production processes that. The chapter then covers how to develop, implement, and validate a. The validation process involves testing and verifying the performance of sterile filters to. Sterile Filter Validation.
From lubrizolcdmo.com
Filter Validation Key Elements for Sterile Drug Product Manufacturing Sterile Filter Validation Validation by sartorius ensures reliable production processes that. Complete your process validation with confidence ®. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. There are eight elements to filter validation: The chapter then covers how to develop, implement, and validate a. The validation process involves testing and. Sterile Filter Validation.
From www.kirikcitarim.com
sterilization热,过滤,辐射的物理方法 Sterile Filter Validation Complete your process validation with confidence ®. The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. Focus on these 3 elements of validation sterile filter master extractables/ plan prove the stream does not adversely impact the filter. There are eight elements to filter validation: The chapter then covers how to develop,. Sterile Filter Validation.
From copure.com.au
Validation Services Copure Sterile Filter Validation The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. There are eight elements to filter validation: The chapter then covers how to develop, implement, and validate a. Validation by sartorius ensures reliable production processes that. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and. Sterile Filter Validation.
From medicaldeviceacademy.com
How to select and help validate the best sterilization method? Sterile Filter Validation Validation by sartorius ensures reliable production processes that. The chapter then covers how to develop, implement, and validate a. There are eight elements to filter validation: Complete your process validation with confidence ®. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. Sterile filters, their properties, manufacture, retention. Sterile Filter Validation.
From www.youtube.com
SterileFiltering Reagents with a Vacuum Filter YouTube Sterile Filter Validation There are eight elements to filter validation: Focus on these 3 elements of validation sterile filter master extractables/ plan prove the stream does not adversely impact the filter. Sterile filters, their properties, manufacture, retention mechanisms, and economics are described. The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. “the integrity of. Sterile Filter Validation.
From eventsget.com
Sterile Filtration of Pharmaceutical Products What you need to know Sterile Filter Validation Focus on these 3 elements of validation sterile filter master extractables/ plan prove the stream does not adversely impact the filter. Sterile filters, their properties, manufacture, retention mechanisms, and economics are described. There are eight elements to filter validation: Validation by sartorius ensures reliable production processes that. The chapter then covers how to develop, implement, and validate a. Filter validation. Sterile Filter Validation.
From microbiologyclass.net
MEMBRANE FILTRATION TECHNIQUE 1 Microbiology Resource Hub Sterile Filter Validation “the integrity of the sterilised filter should be verified before use and should be confirmed immediately after use by an appropriate. Sterile filters, their properties, manufacture, retention mechanisms, and economics are described. There are eight elements to filter validation: Complete your process validation with confidence ®. Focus on these 3 elements of validation sterile filter master extractables/ plan prove the. Sterile Filter Validation.
From dokumen.tips
(PDF) Validation of Sterile Filtration article DOKUMEN.TIPS Sterile Filter Validation There are eight elements to filter validation: Complete your process validation with confidence ®. Focus on these 3 elements of validation sterile filter master extractables/ plan prove the stream does not adversely impact the filter. Sterile filters, their properties, manufacture, retention mechanisms, and economics are described. The chapter then covers how to develop, implement, and validate a. Filter validation is. Sterile Filter Validation.
From www.steris-ims.com
Checking the Integrity of Blue Filters STERIS IMS Sterile Filter Validation “the integrity of the sterilised filter should be verified before use and should be confirmed immediately after use by an appropriate. The chapter then covers how to develop, implement, and validate a. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. Focus on these 3 elements of validation. Sterile Filter Validation.
From www.youtube.com
Key principles and practices for sterilizing filter selection. YouTube Sterile Filter Validation There are eight elements to filter validation: “the integrity of the sterilised filter should be verified before use and should be confirmed immediately after use by an appropriate. Complete your process validation with confidence ®. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. The chapter then covers. Sterile Filter Validation.
From prisystems.com
Sterile Filter Integrity Testing OnSite Factory Trained PRI Bio Sterile Filter Validation Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. Complete your process validation with confidence ®. Validation by sartorius ensures reliable production processes that. There are eight elements to filter validation: The chapter then covers how to develop, implement, and validate a. “the integrity of the sterilised filter. Sterile Filter Validation.
From www.ellab.com
Steam Sterilization Qualification & Validation Healthcare Sterile Filter Validation Complete your process validation with confidence ®. The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. The chapter then covers how to develop, implement, and validate a. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. “the integrity of. Sterile Filter Validation.
From www.youtube.com
Filter Integrity Testing Best Practices YouTube Sterile Filter Validation The chapter then covers how to develop, implement, and validate a. Focus on these 3 elements of validation sterile filter master extractables/ plan prove the stream does not adversely impact the filter. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. Validation by sartorius ensures reliable production processes. Sterile Filter Validation.
From vdocuments.mx
Validation of Sterile Sterile Filter Validation The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. The chapter then covers how to develop, implement, and validate a. “the integrity of the sterilised filter should be verified before use and should be confirmed immediately after use by an appropriate. Sterile filters, their properties, manufacture, retention mechanisms, and economics are. Sterile Filter Validation.
From pharmatreasures.blogspot.com
Pharma Treasures Sterile Filter Validation Question and Answers Sterile Filter Validation Validation by sartorius ensures reliable production processes that. The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. Sterile filters, their properties, manufacture, retention mechanisms, and economics are described. There are eight elements to filter validation: Complete your process validation with confidence ®. Filter validation is done in sterile pharmaceutical manufacturing to. Sterile Filter Validation.
From www.slideshare.net
An Over View of Sterile Filtration Validation A Key Elements for Sterile Filter Validation Validation by sartorius ensures reliable production processes that. “the integrity of the sterilised filter should be verified before use and should be confirmed immediately after use by an appropriate. Complete your process validation with confidence ®. The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. The chapter then covers how to. Sterile Filter Validation.
From www.nelsonlabs.com
Sterilization Validation Test Reuse Device Nelson Labs Sterile Filter Validation Focus on these 3 elements of validation sterile filter master extractables/ plan prove the stream does not adversely impact the filter. The chapter then covers how to develop, implement, and validate a. Validation by sartorius ensures reliable production processes that. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or. Sterile Filter Validation.
From www.nelsonlabs.com
Filter Sterilization Validation Testing Nelson Labs Sterile Filter Validation Validation by sartorius ensures reliable production processes that. Sterile filters, their properties, manufacture, retention mechanisms, and economics are described. There are eight elements to filter validation: “the integrity of the sterilised filter should be verified before use and should be confirmed immediately after use by an appropriate. Focus on these 3 elements of validation sterile filter master extractables/ plan prove. Sterile Filter Validation.
From mdimembrane.com
Sterile Filtration Sterile Filter Validation “the integrity of the sterilised filter should be verified before use and should be confirmed immediately after use by an appropriate. Complete your process validation with confidence ®. The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. Validation by sartorius ensures reliable production processes that. Sterile filters, their properties, manufacture, retention. Sterile Filter Validation.
From microbeonline.com
Moist Heat Sterilization Principle, Advantages, Disadvantages Sterile Filter Validation Validation by sartorius ensures reliable production processes that. The chapter then covers how to develop, implement, and validate a. Focus on these 3 elements of validation sterile filter master extractables/ plan prove the stream does not adversely impact the filter. Complete your process validation with confidence ®. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is. Sterile Filter Validation.
From www.youtube.com
Bacterial Challenge Test Validating SterilizingGrade Filters YouTube Sterile Filter Validation There are eight elements to filter validation: Complete your process validation with confidence ®. The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. Focus on these 3 elements of validation sterile filter master extractables/ plan prove the stream does not adversely impact the filter. The chapter then covers how to develop,. Sterile Filter Validation.
From kerone.com
sterilization Sterile Filter Validation The chapter then covers how to develop, implement, and validate a. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. “the integrity of the sterilised filter should be verified before. Sterile Filter Validation.
From sciencenotes.org
What Is Filtration? Definition and Processes Sterile Filter Validation The chapter then covers how to develop, implement, and validate a. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. Focus on these 3 elements of validation sterile filter master extractables/ plan prove the stream does not adversely impact the filter. Validation by sartorius ensures reliable production processes. Sterile Filter Validation.
From www.docsity.com
Sterile Filtration Validation Best Practices Exams Design Patterns Sterile Filter Validation “the integrity of the sterilised filter should be verified before use and should be confirmed immediately after use by an appropriate. Filter validation is done in sterile pharmaceutical manufacturing to ensure that filter is working properly and sterilizing the material or air. There are eight elements to filter validation: Validation by sartorius ensures reliable production processes that. Complete your process. Sterile Filter Validation.
From www.youtube.com
Filter Sterilization Validations YouTube Sterile Filter Validation The chapter then covers how to develop, implement, and validate a. The validation process involves testing and verifying the performance of sterile filters to ensure they can efectively remove. Complete your process validation with confidence ®. Validation by sartorius ensures reliable production processes that. Sterile filters, their properties, manufacture, retention mechanisms, and economics are described. Filter validation is done in. Sterile Filter Validation.
From www.scribd.com
Filter Validation TrainingBy PALL Sterilization (Microbiology Sterile Filter Validation “the integrity of the sterilised filter should be verified before use and should be confirmed immediately after use by an appropriate. Sterile filters, their properties, manufacture, retention mechanisms, and economics are described. The chapter then covers how to develop, implement, and validate a. Complete your process validation with confidence ®. Validation by sartorius ensures reliable production processes that. The validation. Sterile Filter Validation.