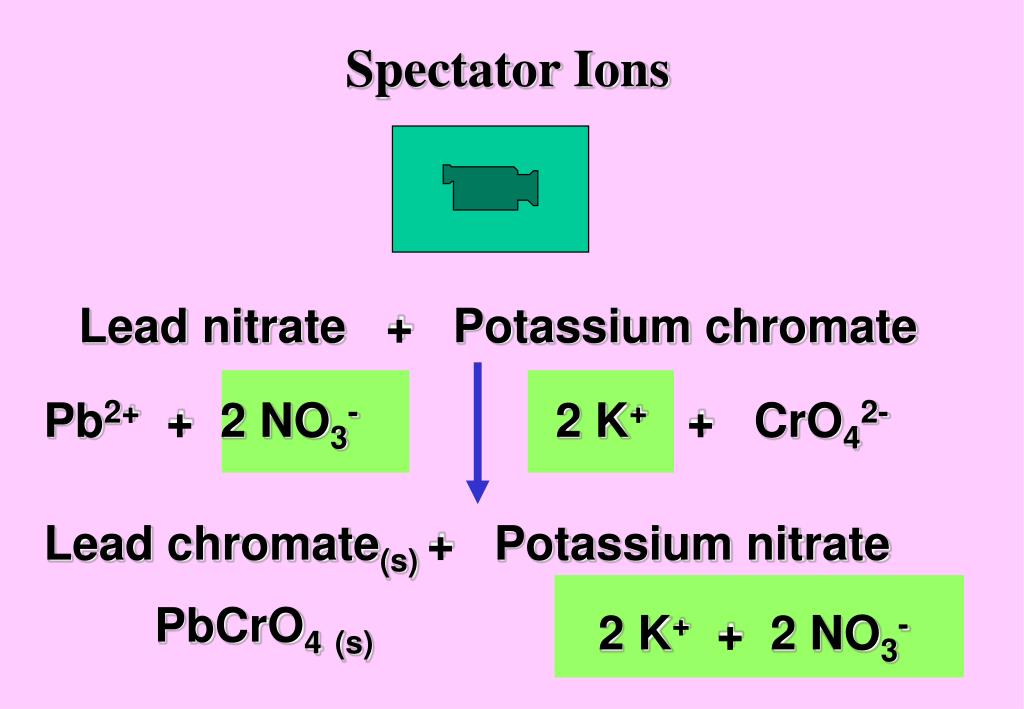
Lead Nitrate Potassium Chromate Equation . When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium. The answer will appear below. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium. Most chromates are as soluble as a brick. enter an equation of a chemical reaction and click 'balance'. P b2+ +cro2− 4 → p bcro4(s) bright yellow precipitate ⏐ ⏐ ⏐ ⏐↓. Always use the upper case for the.
from www.slideserve.com
When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium. Most chromates are as soluble as a brick. P b2+ +cro2− 4 → p bcro4(s) bright yellow precipitate ⏐ ⏐ ⏐ ⏐↓. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. The answer will appear below. Always use the upper case for the. enter an equation of a chemical reaction and click 'balance'. in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium.
PPT General Chemistry II 2302102 PowerPoint Presentation, free
Lead Nitrate Potassium Chromate Equation When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium. let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium. enter an equation of a chemical reaction and click 'balance'. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. P b2+ +cro2− 4 → p bcro4(s) bright yellow precipitate ⏐ ⏐ ⏐ ⏐↓. Always use the upper case for the. When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. Most chromates are as soluble as a brick. The answer will appear below.
From www.chegg.com
Solved Lead nitrate reacts with potassium chromate to Lead Nitrate Potassium Chromate Equation P b2+ +cro2− 4 → p bcro4(s) bright yellow precipitate ⏐ ⏐ ⏐ ⏐↓. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. enter an equation of a chemical reaction and click 'balance'. Most chromates are as soluble as a brick. Always use the upper case for the.. Lead Nitrate Potassium Chromate Equation.
From dxoojemzf.blob.core.windows.net
Sodium Hydroxide Lead Nitrate Balanced Equation at Leonard Griffin blog Lead Nitrate Potassium Chromate Equation Always use the upper case for the. Most chromates are as soluble as a brick. let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. The answer will. Lead Nitrate Potassium Chromate Equation.
From www.numerade.com
SOLVED Write a condensed equation AND net ionic equation for the Lead Nitrate Potassium Chromate Equation enter an equation of a chemical reaction and click 'balance'. P b2+ +cro2− 4 → p bcro4(s) bright yellow precipitate ⏐ ⏐ ⏐ ⏐↓. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. The answer will appear below. let us use a double displacement reaction of lead. Lead Nitrate Potassium Chromate Equation.
From www.numerade.com
SOLVED Lead (II) nitrate + potassium iodide Balanced Chemical Equation Lead Nitrate Potassium Chromate Equation enter an equation of a chemical reaction and click 'balance'. Always use the upper case for the. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium.. Lead Nitrate Potassium Chromate Equation.
From brainly.com
A solution of potassium chromate reacts with a solution of lead(II Lead Nitrate Potassium Chromate Equation k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. enter an equation of a chemical reaction and click 'balance'. When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. let us use a double displacement reaction of lead (ii) nitrate and potassium. Lead Nitrate Potassium Chromate Equation.
From www.chegg.com
Solved 6. Lead nitrate + potassium chromate (Discard in Lead Nitrate Potassium Chromate Equation enter an equation of a chemical reaction and click 'balance'. The answer will appear below. in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium. Most chromates are as soluble as a brick. let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead. Lead Nitrate Potassium Chromate Equation.
From www.coursehero.com
[Solved] lead ( II ) nitrate (aq) + potassium lodide (ag) Formula Lead Nitrate Potassium Chromate Equation Most chromates are as soluble as a brick. The answer will appear below. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. Always use the upper case for the. P b2+ +cro2− 4 →. Lead Nitrate Potassium Chromate Equation.
From www.chegg.com
Solved 13 barium chloride + potassium chromate 14 lead Lead Nitrate Potassium Chromate Equation Always use the upper case for the. P b2+ +cro2− 4 → p bcro4(s) bright yellow precipitate ⏐ ⏐ ⏐ ⏐↓. Most chromates are as soluble as a brick. When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. in order to balance the equation for the double displacement reaction of lead (ii) nitrate. Lead Nitrate Potassium Chromate Equation.
From www.coursehero.com
[Solved] Lead(II) nitrate reacts with potassium chromate to form lead Lead Nitrate Potassium Chromate Equation Always use the upper case for the. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. P b2+ +cro2− 4 → p bcro4(s) bright yellow precipitate ⏐ ⏐ ⏐ ⏐↓. enter an equation. Lead Nitrate Potassium Chromate Equation.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Lead Nitrate Potassium Chromate Equation in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium. When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. The answer will appear below. let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium.. Lead Nitrate Potassium Chromate Equation.
From www.slideserve.com
PPT General Chemistry II 2302102 PowerPoint Presentation, free Lead Nitrate Potassium Chromate Equation When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. let us use a double displacement. Lead Nitrate Potassium Chromate Equation.
From www.slideserve.com
PPT Chapter 4 Part 2 PowerPoint Presentation, free download ID6790428 Lead Nitrate Potassium Chromate Equation Most chromates are as soluble as a brick. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. enter an equation of a chemical reaction and click 'balance'. Always use the upper case for the. When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate. Lead Nitrate Potassium Chromate Equation.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Lead Nitrate Potassium Chromate Equation in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium. enter an equation of a chemical reaction and click 'balance'. When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. Most chromates are as soluble as a brick. let us use a double displacement reaction. Lead Nitrate Potassium Chromate Equation.
From www.numerade.com
SOLVEDWhen a solution of lead(II) nitrate is mixed with a solution of Lead Nitrate Potassium Chromate Equation The answer will appear below. Always use the upper case for the. in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. Most chromates are as soluble as a brick. When lead. Lead Nitrate Potassium Chromate Equation.
From www.showme.com
Formula for potassium chromate Science, Chemistry ShowMe Lead Nitrate Potassium Chromate Equation The answer will appear below. enter an equation of a chemical reaction and click 'balance'. let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. When lead. Lead Nitrate Potassium Chromate Equation.
From www.chegg.com
Solved Reaction of plumbous nitrate with potassium chromate Lead Nitrate Potassium Chromate Equation let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium. Always use the upper case for the. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. in order to balance the equation for the double displacement. Lead Nitrate Potassium Chromate Equation.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free Lead Nitrate Potassium Chromate Equation let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium. P b2+ +cro2− 4 → p bcro4(s) bright yellow precipitate ⏐ ⏐ ⏐ ⏐↓. enter an equation of a chemical reaction and click 'balance'. in order to balance the equation for the double displacement reaction of. Lead Nitrate Potassium Chromate Equation.
From www.numerade.com
SOLVED A solution of potassium chromate reacts with a solution of lead Lead Nitrate Potassium Chromate Equation let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium. The answer will appear below. in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium. Always use the upper case for the. When lead nitrate reacts with potassium chromate formation. Lead Nitrate Potassium Chromate Equation.
From alannahminhardy.blogspot.com
Particle Diagram of Lead Nitrate and Potassium Iodide AlannahminHardy Lead Nitrate Potassium Chromate Equation let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium. P b2+ +cro2− 4 → p bcro4(s) bright yellow precipitate ⏐ ⏐ ⏐ ⏐↓. in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium. The answer will appear below. When. Lead Nitrate Potassium Chromate Equation.
From www.academia.edu
(PDF) Precipitation of lead (II) chromate Folk Narongrit Academia.edu Lead Nitrate Potassium Chromate Equation enter an equation of a chemical reaction and click 'balance'. in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium. When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. Always use the upper case for the. The answer will appear below. P b2+ +cro2− 4. Lead Nitrate Potassium Chromate Equation.
From www.chegg.com
Solved 12. lead (II) nitrate (aq) + potassium chromate (aq) Lead Nitrate Potassium Chromate Equation The answer will appear below. enter an equation of a chemical reaction and click 'balance'. let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium. in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium. k2cro4 + pb(no3)2. Lead Nitrate Potassium Chromate Equation.
From www.coursehero.com
[Solved] Lead(II) nitrate reacts with potassium chromate to form lead Lead Nitrate Potassium Chromate Equation in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium. The answer will appear below. Most chromates are as soluble as a brick. enter an equation of a chemical reaction and click 'balance'. P b2+ +cro2− 4 → p bcro4(s) bright yellow precipitate ⏐ ⏐ ⏐ ⏐↓. When lead nitrate reacts. Lead Nitrate Potassium Chromate Equation.
From www.chegg.com
Solved The precipitation reaction of lead(II) nitrate with Lead Nitrate Potassium Chromate Equation Always use the upper case for the. Most chromates are as soluble as a brick. When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. The answer will appear below. in order to balance. Lead Nitrate Potassium Chromate Equation.
From www.chegg.com
Solved Reaction of plumbous nitrate with potassium chromate. Lead Nitrate Potassium Chromate Equation in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium. let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium. The answer will appear below. Most chromates are as soluble as a brick. Always use the upper case for the.. Lead Nitrate Potassium Chromate Equation.
From www.numerade.com
SOLVED Question 28 If solutions of potassium chromate and sodium Lead Nitrate Potassium Chromate Equation k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. enter an equation of a chemical reaction and click 'balance'. When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. Always use the upper case for the. in order to balance the equation. Lead Nitrate Potassium Chromate Equation.
From slideplayer.com
Chemical Equations Reactants Phase Notation Products Balanced ppt Lead Nitrate Potassium Chromate Equation let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. enter an equation of a chemical reaction and click 'balance'. Always use the upper case for the.. Lead Nitrate Potassium Chromate Equation.
From www.numerade.com
SOLVED Please complete the chart and show calculations. REPORT FOR Lead Nitrate Potassium Chromate Equation enter an equation of a chemical reaction and click 'balance'. When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. The answer will appear below. P b2+ +cro2− 4 → p bcro4(s) bright yellow precipitate ⏐ ⏐ ⏐ ⏐↓. in order to balance the equation for the double displacement reaction of lead (ii). Lead Nitrate Potassium Chromate Equation.
From www.slideshare.net
Chapter 8 Reactions in Aqueous Solution Lead Nitrate Potassium Chromate Equation The answer will appear below. let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium. Always use the upper case for the. P b2+ +cro2− 4 → p bcro4(s) bright yellow precipitate ⏐ ⏐ ⏐ ⏐↓. in order to balance the equation for the double displacement reaction. Lead Nitrate Potassium Chromate Equation.
From www.coursehero.com
[Solved] 3. lead(II) nitrate (aq) + potassium iodide (aq) Observation Lead Nitrate Potassium Chromate Equation k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium. let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium.. Lead Nitrate Potassium Chromate Equation.
From www.slideserve.com
PPT Solubility Rules PowerPoint Presentation, free download ID4934641 Lead Nitrate Potassium Chromate Equation k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium. The answer will appear below. enter an equation of a chemical reaction and click 'balance'. Most chromates. Lead Nitrate Potassium Chromate Equation.
From www.slideserve.com
PPT Chapter 8 Chemical Equations PowerPoint Presentation, free Lead Nitrate Potassium Chromate Equation Always use the upper case for the. Most chromates are as soluble as a brick. When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to produce lead (ii) chromate and potassium. in order to balance the equation for. Lead Nitrate Potassium Chromate Equation.
From slidetodoc.com
Chapter 6 Intro to Chemical Reactions Think of Lead Nitrate Potassium Chromate Equation P b2+ +cro2− 4 → p bcro4(s) bright yellow precipitate ⏐ ⏐ ⏐ ⏐↓. in order to balance the equation for the double displacement reaction of lead (ii) nitrate and potassium. Always use the upper case for the. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. When. Lead Nitrate Potassium Chromate Equation.
From www.teachoo.com
Double Displacement Reaction Definition, Examples, Types Teachoo Lead Nitrate Potassium Chromate Equation Always use the upper case for the. P b2+ +cro2− 4 → p bcro4(s) bright yellow precipitate ⏐ ⏐ ⏐ ⏐↓. The answer will appear below. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. enter an equation of a chemical reaction and click 'balance'. in order. Lead Nitrate Potassium Chromate Equation.
From www.numerade.com
SOLVED Write a balanced equation for the reaction between aqueous lead Lead Nitrate Potassium Chromate Equation Always use the upper case for the. The answer will appear below. enter an equation of a chemical reaction and click 'balance'. When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. Most chromates are as soluble as a brick. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction. Lead Nitrate Potassium Chromate Equation.
From www.youtube.com
007 Potassium Iodide + Lead (II) Nitrate = Lead (II) Iodide Lead Nitrate Potassium Chromate Equation When lead nitrate reacts with potassium chromate formation of lead chromate and potassium nitrate occurs. Most chromates are as soluble as a brick. Always use the upper case for the. k2cro4 + pb(no3)2 = pbcro4 + kno3 is a double displacement (metathesis) reaction where one mole of aqueous potassium. The answer will appear below. let us use a. Lead Nitrate Potassium Chromate Equation.