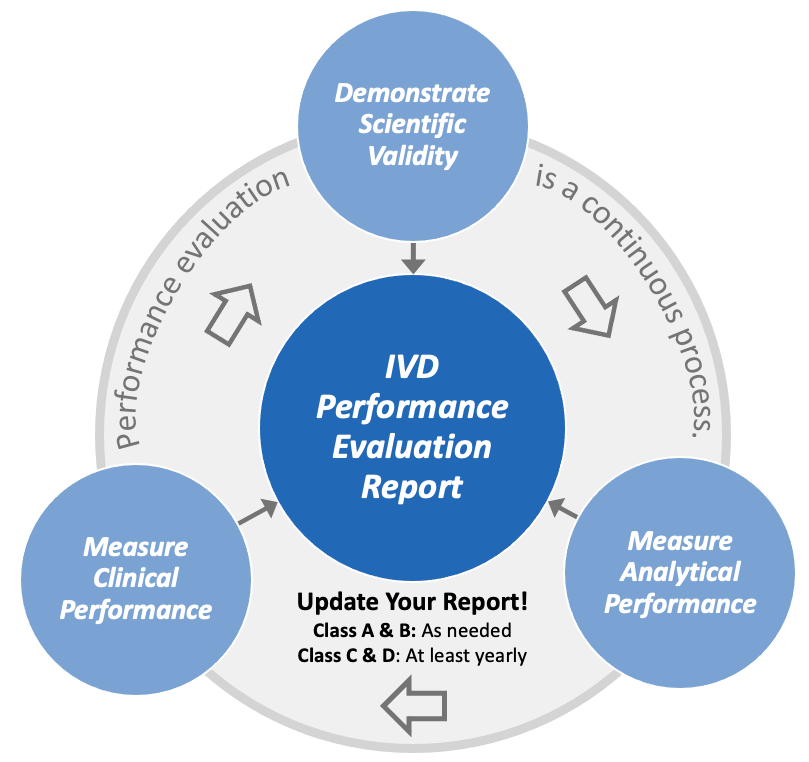
Design Validation Vs Clinical Evaluation . This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. We aim for this common. Clinical evaluation is an integral part of design validation. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). This means the medical devices used for validation have to be built in the production environment,. Your design validation process must include initial production units. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. The clinical evidence, along with other design verification and validation documentation, device description, labelling, risk analysis and.
from www.orielstat.com
Clinical evaluation is an integral part of design validation. This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. We aim for this common. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). Your design validation process must include initial production units. The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. This means the medical devices used for validation have to be built in the production environment,. The clinical evidence, along with other design verification and validation documentation, device description, labelling, risk analysis and.
IVDR Performance Evaluation Report Oriel STAT A MATRIX
Design Validation Vs Clinical Evaluation If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). Clinical evaluation is an integral part of design validation. The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). We aim for this common. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. The clinical evidence, along with other design verification and validation documentation, device description, labelling, risk analysis and. Your design validation process must include initial production units. This means the medical devices used for validation have to be built in the production environment,. This framework includes (1) verification, (2) analytical validation, and (3) clinical validation.
From advisera.com
ISO 9001 Design Verification vs. Design Validation Design Validation Vs Clinical Evaluation We aim for this common. Your design validation process must include initial production units. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also. Design Validation Vs Clinical Evaluation.
From www.slideteam.net
Design Verification And Validation Process Requirement Management Design Validation Vs Clinical Evaluation The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. Clinical evaluation is an integral part of design validation. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part. Design Validation Vs Clinical Evaluation.
From qaconsultinginc.com
Design Verification vs. Design Validation What’s The Difference? QA Design Validation Vs Clinical Evaluation We aim for this common. This means the medical devices used for validation have to be built in the production environment,. The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. The clinical evaluation report (cer) is a central document of the. Design Validation Vs Clinical Evaluation.
From www.researchgate.net
The role of the different disciplinary experts in the V3 process Design Validation Vs Clinical Evaluation We aim for this common. The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. Clinical evaluation is an integral part of design validation. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation. Design Validation Vs Clinical Evaluation.
From www.greenlight.guru
Design Verification & Validation for Medical Devices [Guide] Design Validation Vs Clinical Evaluation If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of.. Design Validation Vs Clinical Evaluation.
From congenius.ch
Navigating Clinical Evaluation for SaMD Congenius Design Validation Vs Clinical Evaluation If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). This means the medical devices used for validation have to be built in the production environment,. We aim for this common. The clinical evidence, along with other design verification and validation documentation, device description, labelling, risk analysis and.. Design Validation Vs Clinical Evaluation.
From www.greenlight.guru
Design Verification & Validation for Medical Devices [Guide] Design Validation Vs Clinical Evaluation We aim for this common. The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. Your design validation process must include initial production units. This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. The clinical evidence, along with other. Design Validation Vs Clinical Evaluation.
From www.researchgate.net
The validation process. The flow chart depicts the series of steps that Design Validation Vs Clinical Evaluation This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. We aim for this common. This means the medical devices used for validation have to be built in the production environment,. The. Design Validation Vs Clinical Evaluation.
From www.researchgate.net
Procedures for Validation of Diagnostic Methods in Clinical Laboratory Design Validation Vs Clinical Evaluation This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is. Design Validation Vs Clinical Evaluation.
From www.researchgate.net
V3 in practice The verification, analytical validation, and clinical Design Validation Vs Clinical Evaluation If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). Clinical evaluation is an integral part of design validation. The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. This. Design Validation Vs Clinical Evaluation.
From qacraft.com
Validation Vs Verification Infographics Design Validation Vs Clinical Evaluation This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. The clinical evidence, along with other design verification and validation documentation, device description, labelling, risk analysis and. This means the medical devices. Design Validation Vs Clinical Evaluation.
From npjdigitalmedcommunity.nature.com
Why V3 (verification, analytical validation, and clinical validation Design Validation Vs Clinical Evaluation The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. Clinical evaluation is an integral part of design validation. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). This means the. Design Validation Vs Clinical Evaluation.
From design.udlvirtual.edu.pe
What Is A Design Validation Plan Design Talk Design Validation Vs Clinical Evaluation The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. This means the medical devices used for validation have to be built in the production environment,. Clinical evaluation is an integral part of design validation. The key differentiator between the two terms is that. Design Validation Vs Clinical Evaluation.
From design.udlvirtual.edu.pe
Design Validation Methods Design Talk Design Validation Vs Clinical Evaluation The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. This means the medical devices used for validation have to be built in the production environment,. The clinical evaluation report. Design Validation Vs Clinical Evaluation.
From www.softwaretestingmaterial.com
What is Verification And Validation In Software Testing Design Validation Vs Clinical Evaluation If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). The clinical evidence, along with other design verification and validation documentation, device description, labelling, risk analysis and. We aim for this common. This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. Your design validation process. Design Validation Vs Clinical Evaluation.
From www.ncbi.nlm.nih.gov
Figure 12, A mapping across three major organizing frameworks for Design Validation Vs Clinical Evaluation Your design validation process must include initial production units. Clinical evaluation is an integral part of design validation. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. The clinical evidence, along with other design verification and validation documentation, device description, labelling, risk analysis. Design Validation Vs Clinical Evaluation.
From www.researchgate.net
shows the main stages of clinical evaluation. The clinical evaluation Design Validation Vs Clinical Evaluation Your design validation process must include initial production units. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. This means the medical devices used for validation have to be built in the production environment,. The key differentiator between the two terms is that. Design Validation Vs Clinical Evaluation.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation, free Design Validation Vs Clinical Evaluation We aim for this common. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. This framework includes (1) verification, (2) analytical. Design Validation Vs Clinical Evaluation.
From www.qualitymeddev.com
Design Verification vs Design Validation What are The Differences Design Validation Vs Clinical Evaluation We aim for this common. Clinical evaluation is an integral part of design validation. The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. Your design validation process must include initial production units. The clinical evidence, along with other design verification and. Design Validation Vs Clinical Evaluation.
From www.orielstat.com
IVDR Performance Evaluation Report Oriel STAT A MATRIX Design Validation Vs Clinical Evaluation If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). Clinical evaluation is an integral part of design validation. This means the medical devices used for validation have to be built in the production environment,. The clinical evaluation report (cer) is a central document of the clinical evaluation. Design Validation Vs Clinical Evaluation.
From www.greenlight.guru
Design Validation vs. Clinical Evaluation What’s the Difference? Design Validation Vs Clinical Evaluation Your design validation process must include initial production units. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. We aim for. Design Validation Vs Clinical Evaluation.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Design Validation Vs Clinical Evaluation This means the medical devices used for validation have to be built in the production environment,. Your design validation process must include initial production units. This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part. Design Validation Vs Clinical Evaluation.
From www.orielstat.com
Medical Device Process Validation Overview & Steps Oriel STAT A MATRIX Design Validation Vs Clinical Evaluation Your design validation process must include initial production units. This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. We aim for this common. This means the medical devices used for validation have to be built in the production environment,. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and. Design Validation Vs Clinical Evaluation.
From www.greenlight.guru
Clinical Evaluation vs Clinical Investigation Difference Explained Design Validation Vs Clinical Evaluation The clinical evidence, along with other design verification and validation documentation, device description, labelling, risk analysis and. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. We aim for this common. This framework includes (1) verification, (2) analytical validation, and (3) clinical validation.. Design Validation Vs Clinical Evaluation.
From www.greenlight.guru
Design Verification & Validation for Medical Devices [Guide] Design Validation Vs Clinical Evaluation This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. The clinical evidence, along with other design verification and validation documentation, device description, labelling, risk analysis and. We aim for. Design Validation Vs Clinical Evaluation.
From www.businessprocessincubator.com
Verification and validation for nextgeneration healthcare Software Design Validation Vs Clinical Evaluation Your design validation process must include initial production units. This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. We aim for this common. The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. The clinical evidence, along with other. Design Validation Vs Clinical Evaluation.
From rs-ness.com
Process Validation Pharma vs. Medical Device RS NESS Design Validation Vs Clinical Evaluation The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. Clinical evaluation is an integral part of design validation. This means the medical devices used for validation have to be built in the production environment,. The clinical evaluation report (cer) is a. Design Validation Vs Clinical Evaluation.
From emmainternational.com
Design Verification vs Design Validation EMMA International Design Validation Vs Clinical Evaluation We aim for this common. Clinical evaluation is an integral part of design validation. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. The key differentiator between the two terms is that design validation is part of design controls and is a required. Design Validation Vs Clinical Evaluation.
From www.emergobyul.com
Analysis of design validation and verification for medical device human Design Validation Vs Clinical Evaluation Your design validation process must include initial production units. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. The clinical evidence, along with other design verification and validation documentation, device description, labelling, risk analysis and.. Design Validation Vs Clinical Evaluation.
From www.semanticscholar.org
Validation and verification of measurement methods in clinical Design Validation Vs Clinical Evaluation This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. Clinical evaluation is an integral part of design validation. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). This means the medical devices used for validation have to be built in the production environment,. The. Design Validation Vs Clinical Evaluation.
From eurotechworld.net
Design Verification and Validation Eurotech Assessment And Design Validation Vs Clinical Evaluation This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. We aim for this common. Your design validation process must include initial production units. This means the medical devices used for validation. Design Validation Vs Clinical Evaluation.
From medicaldevicehq.com
Clinical investigation and clinical evaluation MedicalDeviceHQ Design Validation Vs Clinical Evaluation This means the medical devices used for validation have to be built in the production environment,. The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. The clinical evaluation report. Design Validation Vs Clinical Evaluation.
From www.researchgate.net
Research validation process Download Scientific Diagram Design Validation Vs Clinical Evaluation This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also. Design Validation Vs Clinical Evaluation.
From www.slideteam.net
Production Development With Design Verification And Validation Design Validation Vs Clinical Evaluation If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called v&v). The key differentiator between the two terms is that design validation is part of design controls and is a required part of the product development process prior to. This framework includes (1) verification, (2) analytical validation, and (3). Design Validation Vs Clinical Evaluation.
From clinicalpursuit.com
A Comprehensive Guide to Clinical Data Validation Design Validation Vs Clinical Evaluation This framework includes (1) verification, (2) analytical validation, and (3) clinical validation. The clinical evaluation report (cer) is a central document of the clinical evaluation and of the technical documentation and is a necessary part of evidence of. Clinical evaluation is an integral part of design validation. This means the medical devices used for validation have to be built in. Design Validation Vs Clinical Evaluation.