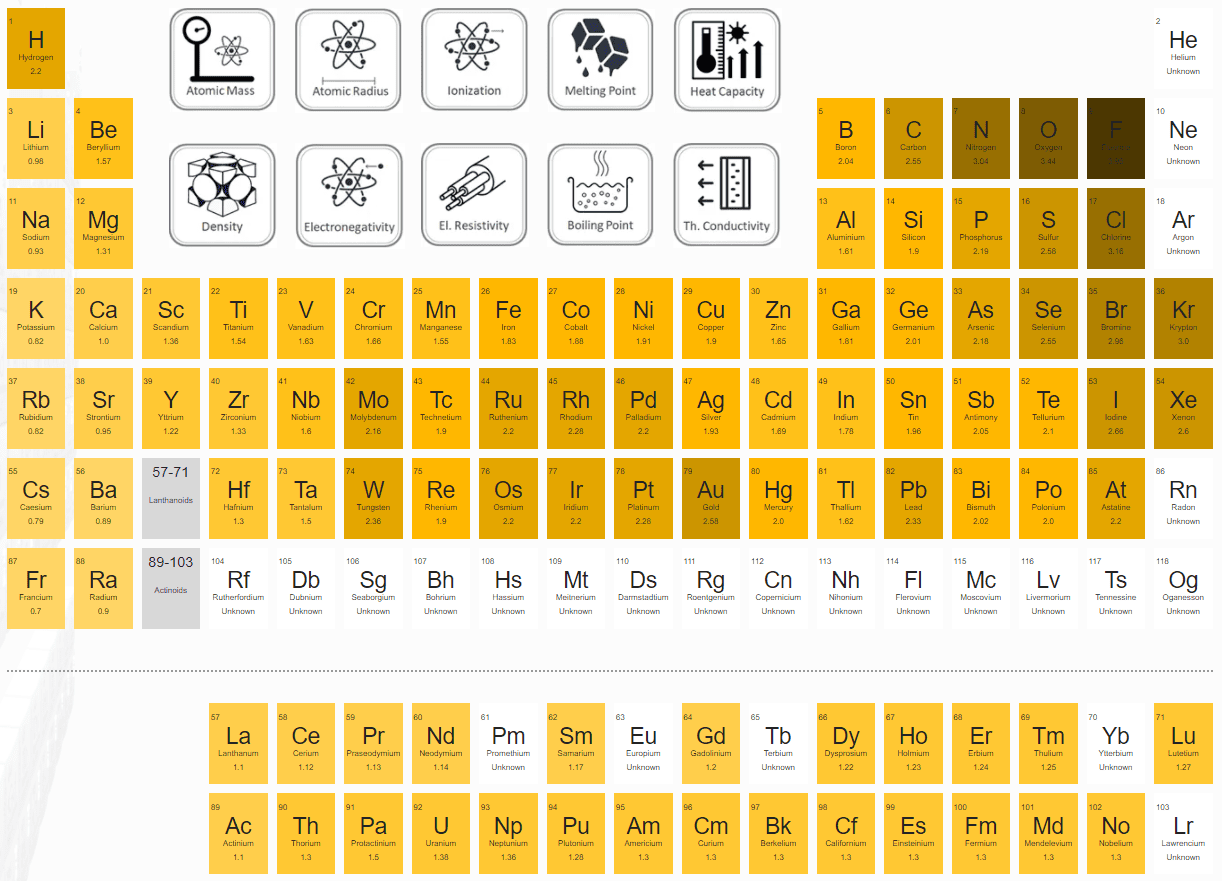
Does Carbon Have Low Electronegativity . 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. It can also be used to predict if the. electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in.
from www.nuclear-power.com
this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. It can also be used to predict if the. electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative.
Electronegativity Pauling Scale
Does Carbon Have Low Electronegativity elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. It can also be used to predict if the. electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Does Carbon Have Low Electronegativity the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. elements with a high electronegativity. Does Carbon Have Low Electronegativity.
From mungfali.com
Electronegativity Trend Explained Does Carbon Have Low Electronegativity this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong. the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. 119 rows electronegativity is used to predict whether a bond. Does Carbon Have Low Electronegativity.
From www.ck12.org
Electronegativity Overview ( Video ) Chemistry CK12 Foundation Does Carbon Have Low Electronegativity electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the hydrogen at the top of the molecule is less. Does Carbon Have Low Electronegativity.
From dona.tompkinscountystructuralracism.org
Electronegativity Values Chart Understanding The Chemistry Of Elements Does Carbon Have Low Electronegativity elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. the hydrogen at the. Does Carbon Have Low Electronegativity.
From material-properties.org
Carbon Periodic Table and Atomic Properties Does Carbon Have Low Electronegativity It can also be used to predict if the. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong. elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. the. Does Carbon Have Low Electronegativity.
From anthonystrendsassignment.weebly.com
Electronegativity Periodic Trends Does Carbon Have Low Electronegativity electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. the electronegativity depends upon a number of factors and in. Does Carbon Have Low Electronegativity.
From wisc.pb.unizin.org
Bonding and Electronegativity (M8Q1) UWMadison Chemistry 103/104 Does Carbon Have Low Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. electrons with low. Does Carbon Have Low Electronegativity.
From ar.inspiredpencil.com
Electronegativity Does Carbon Have Low Electronegativity elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. It can also be used to predict if the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the electronegativity depends upon a number of factors and in particuler as the. Does Carbon Have Low Electronegativity.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Does Carbon Have Low Electronegativity electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong. It can also be used to. Does Carbon Have Low Electronegativity.
From www.slideserve.com
PPT Electronegativity? PowerPoint Presentation, free download ID Does Carbon Have Low Electronegativity the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. elements with a high electronegativity are generally nonmetals and. Does Carbon Have Low Electronegativity.
From lessoncampuspolypide.z5.web.core.windows.net
Electronegativity And Polarity Chart Does Carbon Have Low Electronegativity this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. It can also be used to predict if the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. . Does Carbon Have Low Electronegativity.
From www.slideserve.com
PPT Electronegativity and Polarity PowerPoint Presentation, free Does Carbon Have Low Electronegativity the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. the hydrogen at. Does Carbon Have Low Electronegativity.
From mmerevise.co.uk
Electronegativity & Intermolecular Forces MME Does Carbon Have Low Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It can also be used to predict if the. elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself,. Does Carbon Have Low Electronegativity.
From www.slideserve.com
PPT Properties of Carbon Element PowerPoint Presentation, free Does Carbon Have Low Electronegativity It can also be used to predict if the. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. electrons with low ionization energies have low electronegativities because their nuclei do. Does Carbon Have Low Electronegativity.
From www.reddit.com
Electronegativity/ covalent and ionic bonds r/chemhelp Does Carbon Have Low Electronegativity electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. electrons with low. Does Carbon Have Low Electronegativity.
From www.breakingatom.com
Electronegativity of the Elements Does Carbon Have Low Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong. the electronegativity depends upon a number. Does Carbon Have Low Electronegativity.
From sciencetrends.com
Electronegativity Chart Science Trends Does Carbon Have Low Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. It can also be. Does Carbon Have Low Electronegativity.
From www.nuclear-power.com
Electronegativity Pauling Scale Does Carbon Have Low Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. electrons with low ionization. Does Carbon Have Low Electronegativity.
From www.youtube.com
Electronegativity, Basic Introduction, Periodic Trends Which Element Does Carbon Have Low Electronegativity the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. elements with a. Does Carbon Have Low Electronegativity.
From general.chemistrysteps.com
Electronegativity and Bond Polarity Chemistry Steps Does Carbon Have Low Electronegativity this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. It can also be used to predict if the. elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. . Does Carbon Have Low Electronegativity.
From iperiodictable.com
What is Electronegativity Chart List of Electronegativity [PDF Does Carbon Have Low Electronegativity this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. electrons with low ionization energies have low electronegativities because. Does Carbon Have Low Electronegativity.
From www.nuclear-power.com
Carbon Electron Affinity Electronegativity Ionization Energy of Does Carbon Have Low Electronegativity electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong. the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. electronegativity (pauling scale) the tendency. Does Carbon Have Low Electronegativity.
From therealgroupichem.weebly.com
Electronegativity Group i Chemistry(iiiescuro) Does Carbon Have Low Electronegativity the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. 119 rows electronegativity is used to predict whether a. Does Carbon Have Low Electronegativity.
From mavink.com
Periodic Table With Electronegativity Values Does Carbon Have Low Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. It can also be used. Does Carbon Have Low Electronegativity.
From www.numerade.com
SOLVED Carbon has a electronegativity of 2.5 and oxygen has an Does Carbon Have Low Electronegativity electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. 119 rows electronegativity is used to predict whether a. Does Carbon Have Low Electronegativity.
From mavink.com
Printable Electronegativity Table Does Carbon Have Low Electronegativity It can also be used to predict if the. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong. the hydrogen at the top of the molecule is less electronegative than carbon and so. Does Carbon Have Low Electronegativity.
From www.pinterest.co.uk
Illustration about Periodic table of elements with electronegativity Does Carbon Have Low Electronegativity electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. It can also be used to predict if the. the hydrogen at the top of the molecule is less electronegative than carbon. Does Carbon Have Low Electronegativity.
From www.thoughtco.com
Printable Periodic Table of the Elements Electronegativity Does Carbon Have Low Electronegativity electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. this is why. Does Carbon Have Low Electronegativity.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID6836286 Does Carbon Have Low Electronegativity electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. It can also be used to predict if the. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. . Does Carbon Have Low Electronegativity.
From sciencenotes.org
List of Electronegativity Values of the Elements Does Carbon Have Low Electronegativity electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. the electronegativity depends upon a number of factors and. Does Carbon Have Low Electronegativity.
From www.britannica.com
Chemical compound Trends in the chemical properties of the elements Does Carbon Have Low Electronegativity this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. It can also be used to predict if the. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. . Does Carbon Have Low Electronegativity.
From dreamstime.com
Electronegativity Periodic Table Stock Image Image 38153931 Does Carbon Have Low Electronegativity electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. It can also be used to. Does Carbon Have Low Electronegativity.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Does Carbon Have Low Electronegativity the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. 119 rows electronegativity. Does Carbon Have Low Electronegativity.
From socratic.org
What would cause an atom to have a low electronegativity value? Socratic Does Carbon Have Low Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. this is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically. elements with a high electronegativity are generally nonmetals and. Does Carbon Have Low Electronegativity.
From www.researchgate.net
The role of the electronegativity in the charge of the carbon atom. V Does Carbon Have Low Electronegativity elements with a high electronegativity are generally nonmetals and electrical insulators and tend to behave as oxidants in. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative. electrons with low ionization. Does Carbon Have Low Electronegativity.