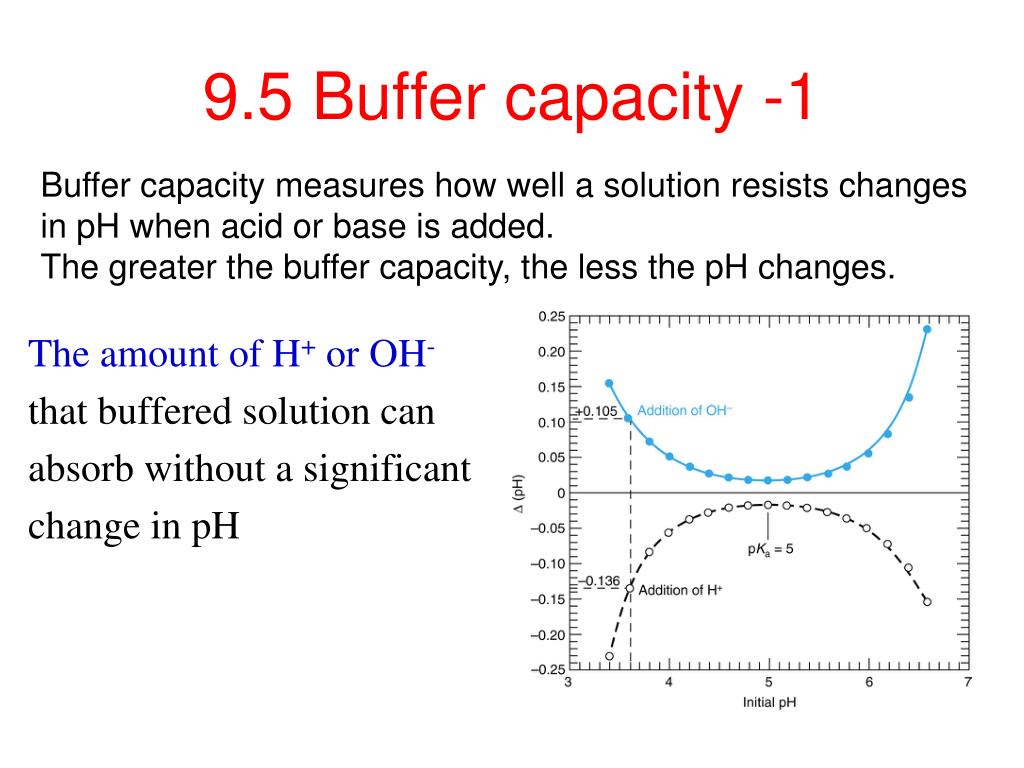
Buffer Capacity Vs Dilution . Buffers are characterized by their ph range and buffer capacity. The chemical composition of a buffer solution usually entails a weak. However the effect is really. Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. They are diluted to the. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio.
from www.slideserve.com
If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. The chemical composition of a buffer solution usually entails a weak. They are diluted to the. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. Buffers are characterized by their ph range and buffer capacity. Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. However the effect is really.
PPT Chapter 8 & 9 PowerPoint Presentation, free download ID991133
Buffer Capacity Vs Dilution Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. Buffers are characterized by their ph range and buffer capacity. However the effect is really. Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. The chemical composition of a buffer solution usually entails a weak. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. They are diluted to the. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint Buffer Capacity Vs Dilution The chemical composition of a buffer solution usually entails a weak. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. However the effect is really. Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. If you look. Buffer Capacity Vs Dilution.
From ar.inspiredpencil.com
Buffer Capacity Buffer Capacity Vs Dilution Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. However the effect is really. Buffers are characterized by their ph. Buffer Capacity Vs Dilution.
From pdfprof.com
buffer capacity experiment procedure Buffer Capacity Vs Dilution If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. However the effect is really. They are diluted to the. Buffers are characterized by their ph. Buffer Capacity Vs Dilution.
From www.mdpi.com
IJMS Free FullText A Study of the Buffer Capacity of Buffer Capacity Vs Dilution The chemical composition of a buffer solution usually entails a weak. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will.. Buffer Capacity Vs Dilution.
From www.researchgate.net
4 questions with answers in BUFFERS Science topic Buffer Capacity Vs Dilution They are diluted to the. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. Buffers are characterized by their ph range and buffer capacity. However the effect is really. The chemical composition of a buffer solution usually entails a weak. If you look at the buffer formula,. Buffer Capacity Vs Dilution.
From www.slideserve.com
PPT Chapter Two Water The Solvent for Biochemical Reactions Buffer Capacity Vs Dilution Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph. Buffer Capacity Vs Dilution.
From www.researchgate.net
pH change in different concentrations of phosphate buffer with 5 mM Buffer Capacity Vs Dilution Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph. Buffer Capacity Vs Dilution.
From www.studypool.com
SOLUTION 4 buffering capacity Studypool Buffer Capacity Vs Dilution However the effect is really. They are diluted to the. Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. If you look at the buffer formula, ph =. Buffer Capacity Vs Dilution.
From www.youtube.com
Buffer capacity; making a buffer with a given pH value YouTube Buffer Capacity Vs Dilution However the effect is really. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. Buffers consist of an acid and. Buffer Capacity Vs Dilution.
From university.pressbooks.pub
15.6 Buffers Chemistry Fundamentals Buffer Capacity Vs Dilution However the effect is really. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. They are diluted to the. Buffers are characterized by their ph range and buffer capacity. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before. Buffer Capacity Vs Dilution.
From ar.inspiredpencil.com
Buffer Capacity Buffer Capacity Vs Dilution If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. However the effect is really. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. Buffers are characterized by their ph range and buffer capacity. Buffers. Buffer Capacity Vs Dilution.
From www.youtube.com
Buffer Range and Buffer Capacity.mp4 YouTube Buffer Capacity Vs Dilution Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. However the effect is really. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. Buffer solutions have a working ph range and capacity which dictate how much acid/base. Buffer Capacity Vs Dilution.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free Buffer Capacity Vs Dilution Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. Buffers are characterized by their ph range and buffer capacity. Buffers. Buffer Capacity Vs Dilution.
From ar.inspiredpencil.com
Buffer Capacity Buffer Capacity Vs Dilution A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. Buffers are characterized by their ph range and buffer capacity. However the effect is really. They. Buffer Capacity Vs Dilution.
From pharmacyscope.com
How do you calculate buffer capacity? Pharmacy Scope Buffer Capacity Vs Dilution Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. However the effect is really. If you look at the buffer formula, ph =. Buffer Capacity Vs Dilution.
From www.slideserve.com
PPT Buffers & Buffer Capacity PowerPoint Presentation ID5949383 Buffer Capacity Vs Dilution Buffers are characterized by their ph range and buffer capacity. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. However. Buffer Capacity Vs Dilution.
From studylib.net
Buffer Capacity Buffer Capacity Vs Dilution Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. Buffers are characterized by their ph range and buffer capacity. Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. The chemical composition of. Buffer Capacity Vs Dilution.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free Buffer Capacity Vs Dilution If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. A buffer is a compound that resists changes in ph when. Buffer Capacity Vs Dilution.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Buffer Capacity Vs Dilution Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. They are diluted to the. Buffers are characterized by their ph range and buffer capacity. However the effect is really. The chemical composition of a buffer solution usually entails a weak. A buffer is a compound that resists changes in ph. Buffer Capacity Vs Dilution.
From relationshipbetween.com
Difference Between Buffer Action And Buffer Capacity Relationship Between Buffer Capacity Vs Dilution However the effect is really. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. Buffer solutions have a working ph range and capacity which dictate how much acid/base. Buffer Capacity Vs Dilution.
From ppt-online.org
Solutions. Acidbase equilibrium in biological systems презентация онлайн Buffer Capacity Vs Dilution Buffers are characterized by their ph range and buffer capacity. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. However. Buffer Capacity Vs Dilution.
From dandkmotorsports.com
Tris Hcl Buffer Ph 8 5 Recipe Dandk Organizer Buffer Capacity Vs Dilution Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. If you look at the buffer formula, ph = pka + lg [salt]/ [acid],. Buffer Capacity Vs Dilution.
From www.slideserve.com
PPT Chapter 8 & 9 PowerPoint Presentation, free download ID991133 Buffer Capacity Vs Dilution However the effect is really. The chemical composition of a buffer solution usually entails a weak. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and. Buffer Capacity Vs Dilution.
From www.slideserve.com
PPT Chapter 13 Applications of Aqueous Equilibria PowerPoint Buffer Capacity Vs Dilution However the effect is really. They are diluted to the. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. Buffers are characterized by their ph range and buffer capacity. Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic. Buffer Capacity Vs Dilution.
From www.researchgate.net
Buffering capacity β of (a) water, (b) ammonia or ammonium, and (c Buffer Capacity Vs Dilution Buffers are characterized by their ph range and buffer capacity. Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. However the effect is really. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it. Buffer Capacity Vs Dilution.
From saylordotorg.github.io
Buffers Buffer Capacity Vs Dilution Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph. Buffer Capacity Vs Dilution.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Buffer Capacity Vs Dilution If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. Buffers are characterized by their ph range and buffer capacity. A buffer is a compound that resists changes in. Buffer Capacity Vs Dilution.
From www.reddit.com
Is it just me or does the bottom right graph contradict the Buffer Capacity Vs Dilution However the effect is really. They are diluted to the. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. The. Buffer Capacity Vs Dilution.
From ar.inspiredpencil.com
Buffer Capacity Buffer Capacity Vs Dilution Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. The chemical composition of a buffer solution usually entails a weak. If you look. Buffer Capacity Vs Dilution.
From www.toppr.com
The buffer capacity (beta) for a weak acid (A) conjugate base (B Buffer Capacity Vs Dilution A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. However the effect is really. If you look at the buffer. Buffer Capacity Vs Dilution.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint Buffer Capacity Vs Dilution The chemical composition of a buffer solution usually entails a weak. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. Buffers are characterized by their ph range and buffer capacity. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not. Buffer Capacity Vs Dilution.
From www.vrogue.co
What Is Serial Dilution Method And How To Calculate S vrogue.co Buffer Capacity Vs Dilution However the effect is really. The chemical composition of a buffer solution usually entails a weak. A buffer is a compound that resists changes in ph when a limited amount of acid or base is added to it. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio.. Buffer Capacity Vs Dilution.
From www.thetechedvocate.org
How to calculate buffer capacity The Tech Edvocate Buffer Capacity Vs Dilution The chemical composition of a buffer solution usually entails a weak. Buffer solutions have a working ph range and capacity which dictate how much acid/base can be neutralized before ph changes, and the amount by which it will. Buffers are characterized by their ph range and buffer capacity. However the effect is really. They are diluted to the. A buffer. Buffer Capacity Vs Dilution.
From animalia-life.club
Phosphate Buffer System Equation Buffer Capacity Vs Dilution They are diluted to the. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. The chemical composition of a buffer solution usually entails a weak. Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. A buffer is. Buffer Capacity Vs Dilution.
From facts.net
13 Enigmatic Facts About Buffer Capacity Buffer Capacity Vs Dilution Buffers consist of an acid and its conjugate base, such as carbonate and bicarbonate, or acetate and acetic acid. They are diluted to the. If you look at the buffer formula, ph = pka + lg [salt]/ [acid], dilution does not affect the [salt]/ [acid] ratio. The chemical composition of a buffer solution usually entails a weak. However the effect. Buffer Capacity Vs Dilution.