Examples Of Liquid Saturated Solution . Explain how supersaturated solutions are created. A saturated solution is a chemical solution that contains the highest bound solvent level. A saturated solution is one that does not admit the dissolution of more solute. When pressure decreases by opening the container, the solubility of. Under some circumstances it is possible to prepare a solution which behaves anomalously and contains more solute than a saturated. In these drinks, water is a solvent and carbon is bombarded as a. As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates the maximum amount of a. Beverages are one of the most widely used and loved saturated solutions. In other words, it is a solution in which the maximum. Here are some familiar examples: Everyday examples of saturated solution. In a saturated solution, the extra solution will not. A soda is a saturated solution of carbon dioxide in water.
from www.teachoo.com
In other words, it is a solution in which the maximum. A saturated solution is a chemical solution that contains the highest bound solvent level. In these drinks, water is a solvent and carbon is bombarded as a. A saturated solution is one that does not admit the dissolution of more solute. Beverages are one of the most widely used and loved saturated solutions. In a saturated solution, the extra solution will not. Explain how supersaturated solutions are created. When pressure decreases by opening the container, the solubility of. Everyday examples of saturated solution. Here are some familiar examples:
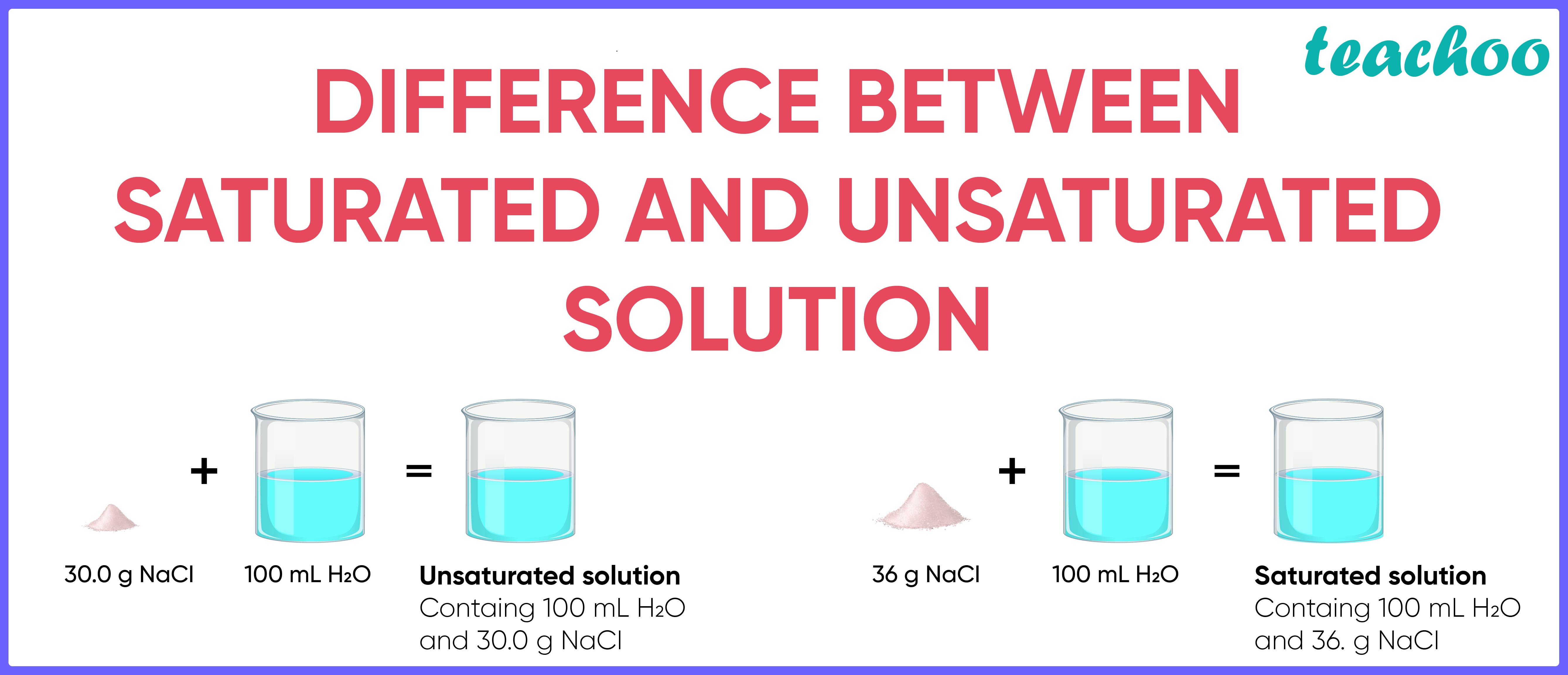
Difference between Saturated and Unsaturated Solution Teachoo
Examples Of Liquid Saturated Solution Here are some familiar examples: When pressure decreases by opening the container, the solubility of. In these drinks, water is a solvent and carbon is bombarded as a. Beverages are one of the most widely used and loved saturated solutions. In a saturated solution, the extra solution will not. Everyday examples of saturated solution. As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates the maximum amount of a. A saturated solution is one that does not admit the dissolution of more solute. Under some circumstances it is possible to prepare a solution which behaves anomalously and contains more solute than a saturated. A saturated solution is a chemical solution that contains the highest bound solvent level. In other words, it is a solution in which the maximum. A soda is a saturated solution of carbon dioxide in water. Explain how supersaturated solutions are created. Here are some familiar examples:
From www.yaclass.in
Types of solutions Based on the amount of the solute — lesson. Science Examples Of Liquid Saturated Solution As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates the maximum amount of a. When pressure decreases by opening the container, the solubility of. Everyday examples of saturated solution. A saturated solution is one that does not admit the dissolution of more solute. Beverages are one of the most widely. Examples Of Liquid Saturated Solution.
From www.slideserve.com
PPT Liquid dosage forms PowerPoint Presentation ID4329709 Examples Of Liquid Saturated Solution In a saturated solution, the extra solution will not. Explain how supersaturated solutions are created. In these drinks, water is a solvent and carbon is bombarded as a. As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates the maximum amount of a. When pressure decreases by opening the container, the. Examples Of Liquid Saturated Solution.
From ar.inspiredpencil.com
Saturated Solution Examples Of Liquid Saturated Solution When pressure decreases by opening the container, the solubility of. A saturated solution is a chemical solution that contains the highest bound solvent level. A saturated solution is one that does not admit the dissolution of more solute. Beverages are one of the most widely used and loved saturated solutions. As discussed in the previous section of this chapter, a. Examples Of Liquid Saturated Solution.
From www.slideserve.com
PPT Solubility PowerPoint Presentation ID3105812 Examples Of Liquid Saturated Solution As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates the maximum amount of a. Everyday examples of saturated solution. In a saturated solution, the extra solution will not. Beverages are one of the most widely used and loved saturated solutions. When pressure decreases by opening the container, the solubility of.. Examples Of Liquid Saturated Solution.
From quizizz.com
Saturated Solutions Science Quizizz Examples Of Liquid Saturated Solution Under some circumstances it is possible to prepare a solution which behaves anomalously and contains more solute than a saturated. As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates the maximum amount of a. Here are some familiar examples: A saturated solution is a chemical solution that contains the highest. Examples Of Liquid Saturated Solution.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID3105812 Examples Of Liquid Saturated Solution Here are some familiar examples: In other words, it is a solution in which the maximum. A saturated solution is one that does not admit the dissolution of more solute. Explain how supersaturated solutions are created. As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates the maximum amount of a.. Examples Of Liquid Saturated Solution.
From www.slideshare.net
Water and solutions Examples Of Liquid Saturated Solution A soda is a saturated solution of carbon dioxide in water. When pressure decreases by opening the container, the solubility of. In other words, it is a solution in which the maximum. A saturated solution is a chemical solution that contains the highest bound solvent level. In a saturated solution, the extra solution will not. In these drinks, water is. Examples Of Liquid Saturated Solution.
From www.slideserve.com
PPT Chp 13. SOLUTIONS PowerPoint Presentation, free download ID1098965 Examples Of Liquid Saturated Solution Explain how supersaturated solutions are created. In these drinks, water is a solvent and carbon is bombarded as a. Beverages are one of the most widely used and loved saturated solutions. A saturated solution is a chemical solution that contains the highest bound solvent level. Under some circumstances it is possible to prepare a solution which behaves anomalously and contains. Examples Of Liquid Saturated Solution.
From www.slideserve.com
PPT Unit 7 Solution Chemistry Chapter 13 PowerPoint Presentation Examples Of Liquid Saturated Solution Under some circumstances it is possible to prepare a solution which behaves anomalously and contains more solute than a saturated. A soda is a saturated solution of carbon dioxide in water. As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates the maximum amount of a. In these drinks, water is. Examples Of Liquid Saturated Solution.
From classnotes.org.in
Saturated Solution Class 6, Separation of Substances Examples Of Liquid Saturated Solution A soda is a saturated solution of carbon dioxide in water. A saturated solution is one that does not admit the dissolution of more solute. A saturated solution is a chemical solution that contains the highest bound solvent level. Beverages are one of the most widely used and loved saturated solutions. Under some circumstances it is possible to prepare a. Examples Of Liquid Saturated Solution.
From amirecmccullough.blogspot.com
What is Saturated Solution AmirecMccullough Examples Of Liquid Saturated Solution Explain how supersaturated solutions are created. In other words, it is a solution in which the maximum. As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates the maximum amount of a. A saturated solution is a chemical solution that contains the highest bound solvent level. Here are some familiar examples:. Examples Of Liquid Saturated Solution.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation Examples Of Liquid Saturated Solution When pressure decreases by opening the container, the solubility of. In a saturated solution, the extra solution will not. A saturated solution is a chemical solution that contains the highest bound solvent level. In other words, it is a solution in which the maximum. Everyday examples of saturated solution. Beverages are one of the most widely used and loved saturated. Examples Of Liquid Saturated Solution.
From amirecmccullough.blogspot.com
What is Saturated Solution AmirecMccullough Examples Of Liquid Saturated Solution A saturated solution is a chemical solution that contains the highest bound solvent level. Beverages are one of the most widely used and loved saturated solutions. Here are some familiar examples: As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates the maximum amount of a. In other words, it is. Examples Of Liquid Saturated Solution.
From www.echemi.com
What is the difference between a saturated and a super saturated Examples Of Liquid Saturated Solution In other words, it is a solution in which the maximum. A saturated solution is one that does not admit the dissolution of more solute. A saturated solution is a chemical solution that contains the highest bound solvent level. Everyday examples of saturated solution. Explain how supersaturated solutions are created. Under some circumstances it is possible to prepare a solution. Examples Of Liquid Saturated Solution.
From amirecmccullough.blogspot.com
What is Saturated Solution AmirecMccullough Examples Of Liquid Saturated Solution A saturated solution is one that does not admit the dissolution of more solute. In these drinks, water is a solvent and carbon is bombarded as a. A soda is a saturated solution of carbon dioxide in water. A saturated solution is a chemical solution that contains the highest bound solvent level. Explain how supersaturated solutions are created. Here are. Examples Of Liquid Saturated Solution.
From www.teachoo.com
Solution Definition, Types, Properties Chemistry Teachoo Examples Of Liquid Saturated Solution As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates the maximum amount of a. When pressure decreases by opening the container, the solubility of. A saturated solution is a chemical solution that contains the highest bound solvent level. A soda is a saturated solution of carbon dioxide in water. Beverages. Examples Of Liquid Saturated Solution.
From study.com
Saturated Solution Definition & Examples Lesson Examples Of Liquid Saturated Solution Here are some familiar examples: A saturated solution is one that does not admit the dissolution of more solute. Beverages are one of the most widely used and loved saturated solutions. A saturated solution is a chemical solution that contains the highest bound solvent level. Under some circumstances it is possible to prepare a solution which behaves anomalously and contains. Examples Of Liquid Saturated Solution.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Examples Of Liquid Saturated Solution In a saturated solution, the extra solution will not. In these drinks, water is a solvent and carbon is bombarded as a. A saturated solution is a chemical solution that contains the highest bound solvent level. A saturated solution is one that does not admit the dissolution of more solute. Beverages are one of the most widely used and loved. Examples Of Liquid Saturated Solution.
From examples.yourdictionary.com
Examples of Saturated Solution YourDictionary Examples Of Liquid Saturated Solution In these drinks, water is a solvent and carbon is bombarded as a. Everyday examples of saturated solution. A soda is a saturated solution of carbon dioxide in water. A saturated solution is one that does not admit the dissolution of more solute. A saturated solution is a chemical solution that contains the highest bound solvent level. As discussed in. Examples Of Liquid Saturated Solution.
From sciencenotes.org
Saturated Solution Definition in Chemistry Examples Of Liquid Saturated Solution Here are some familiar examples: A soda is a saturated solution of carbon dioxide in water. Beverages are one of the most widely used and loved saturated solutions. A saturated solution is one that does not admit the dissolution of more solute. In other words, it is a solution in which the maximum. A saturated solution is a chemical solution. Examples Of Liquid Saturated Solution.
From ar.inspiredpencil.com
Saturated Solution Examples Of Liquid Saturated Solution In these drinks, water is a solvent and carbon is bombarded as a. Explain how supersaturated solutions are created. As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates the maximum amount of a. When pressure decreases by opening the container, the solubility of. A soda is a saturated solution of. Examples Of Liquid Saturated Solution.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Examples Of Liquid Saturated Solution Beverages are one of the most widely used and loved saturated solutions. As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates the maximum amount of a. A saturated solution is a chemical solution that contains the highest bound solvent level. Explain how supersaturated solutions are created. In other words, it. Examples Of Liquid Saturated Solution.
From www.geeksforgeeks.org
Concentration of Solution Definition, Formulas & Solved Examples Examples Of Liquid Saturated Solution Beverages are one of the most widely used and loved saturated solutions. In these drinks, water is a solvent and carbon is bombarded as a. When pressure decreases by opening the container, the solubility of. Under some circumstances it is possible to prepare a solution which behaves anomalously and contains more solute than a saturated. In other words, it is. Examples Of Liquid Saturated Solution.
From sciencemsqblog8.blogspot.com
Science8 Semester 2,Chapter 4 Mixtures Examples Of Liquid Saturated Solution Explain how supersaturated solutions are created. In a saturated solution, the extra solution will not. A soda is a saturated solution of carbon dioxide in water. Here are some familiar examples: When pressure decreases by opening the container, the solubility of. Under some circumstances it is possible to prepare a solution which behaves anomalously and contains more solute than a. Examples Of Liquid Saturated Solution.
From sciencenotes.org
Unsaturated Solution Definition and Examples in Chemistry Examples Of Liquid Saturated Solution In other words, it is a solution in which the maximum. Everyday examples of saturated solution. When pressure decreases by opening the container, the solubility of. In these drinks, water is a solvent and carbon is bombarded as a. Here are some familiar examples: In a saturated solution, the extra solution will not. A saturated solution is one that does. Examples Of Liquid Saturated Solution.
From sciencenotes.org
Supersaturated Solution Definition and Examples Examples Of Liquid Saturated Solution When pressure decreases by opening the container, the solubility of. Here are some familiar examples: Everyday examples of saturated solution. A saturated solution is a chemical solution that contains the highest bound solvent level. A saturated solution is one that does not admit the dissolution of more solute. A soda is a saturated solution of carbon dioxide in water. Beverages. Examples Of Liquid Saturated Solution.
From www.slideshare.net
Online Solutions Examples Of Liquid Saturated Solution A saturated solution is one that does not admit the dissolution of more solute. In a saturated solution, the extra solution will not. Beverages are one of the most widely used and loved saturated solutions. In other words, it is a solution in which the maximum. A soda is a saturated solution of carbon dioxide in water. Here are some. Examples Of Liquid Saturated Solution.
From www.slideserve.com
PPT Chapter 13 Properties of Solutions PowerPoint Presentation, free Examples Of Liquid Saturated Solution As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates the maximum amount of a. Explain how supersaturated solutions are created. A saturated solution is a chemical solution that contains the highest bound solvent level. When pressure decreases by opening the container, the solubility of. In a saturated solution, the extra. Examples Of Liquid Saturated Solution.
From www.thoughtco.com
Saturated Solution Definition and Examples Examples Of Liquid Saturated Solution In a saturated solution, the extra solution will not. Under some circumstances it is possible to prepare a solution which behaves anomalously and contains more solute than a saturated. In these drinks, water is a solvent and carbon is bombarded as a. Explain how supersaturated solutions are created. A saturated solution is one that does not admit the dissolution of. Examples Of Liquid Saturated Solution.
From ar.inspiredpencil.com
Saturated Solution Examples Of Liquid Saturated Solution Under some circumstances it is possible to prepare a solution which behaves anomalously and contains more solute than a saturated. Beverages are one of the most widely used and loved saturated solutions. A saturated solution is a chemical solution that contains the highest bound solvent level. A soda is a saturated solution of carbon dioxide in water. When pressure decreases. Examples Of Liquid Saturated Solution.
From www.teachoo.com
Difference between Saturated and Unsaturated Solution Teachoo Examples Of Liquid Saturated Solution Beverages are one of the most widely used and loved saturated solutions. Under some circumstances it is possible to prepare a solution which behaves anomalously and contains more solute than a saturated. A saturated solution is a chemical solution that contains the highest bound solvent level. Here are some familiar examples: In other words, it is a solution in which. Examples Of Liquid Saturated Solution.
From www.slideserve.com
PPT Introduction to Solutions PowerPoint Presentation, free download Examples Of Liquid Saturated Solution In these drinks, water is a solvent and carbon is bombarded as a. A saturated solution is a chemical solution that contains the highest bound solvent level. As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates the maximum amount of a. In a saturated solution, the extra solution will not.. Examples Of Liquid Saturated Solution.
From www.slideserve.com
PPT Introduction to Solutions PowerPoint Presentation, free download Examples Of Liquid Saturated Solution A soda is a saturated solution of carbon dioxide in water. A saturated solution is a chemical solution that contains the highest bound solvent level. Everyday examples of saturated solution. In other words, it is a solution in which the maximum. As discussed in the previous section of this chapter, a solubility limit is expressed as a ratio that relates. Examples Of Liquid Saturated Solution.
From gamesmartz.com
Saturated Solution Definition & Image GameSmartz Examples Of Liquid Saturated Solution A saturated solution is one that does not admit the dissolution of more solute. Under some circumstances it is possible to prepare a solution which behaves anomalously and contains more solute than a saturated. Here are some familiar examples: A soda is a saturated solution of carbon dioxide in water. Explain how supersaturated solutions are created. In these drinks, water. Examples Of Liquid Saturated Solution.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID4638445 Examples Of Liquid Saturated Solution A soda is a saturated solution of carbon dioxide in water. In other words, it is a solution in which the maximum. In these drinks, water is a solvent and carbon is bombarded as a. A saturated solution is a chemical solution that contains the highest bound solvent level. Under some circumstances it is possible to prepare a solution which. Examples Of Liquid Saturated Solution.