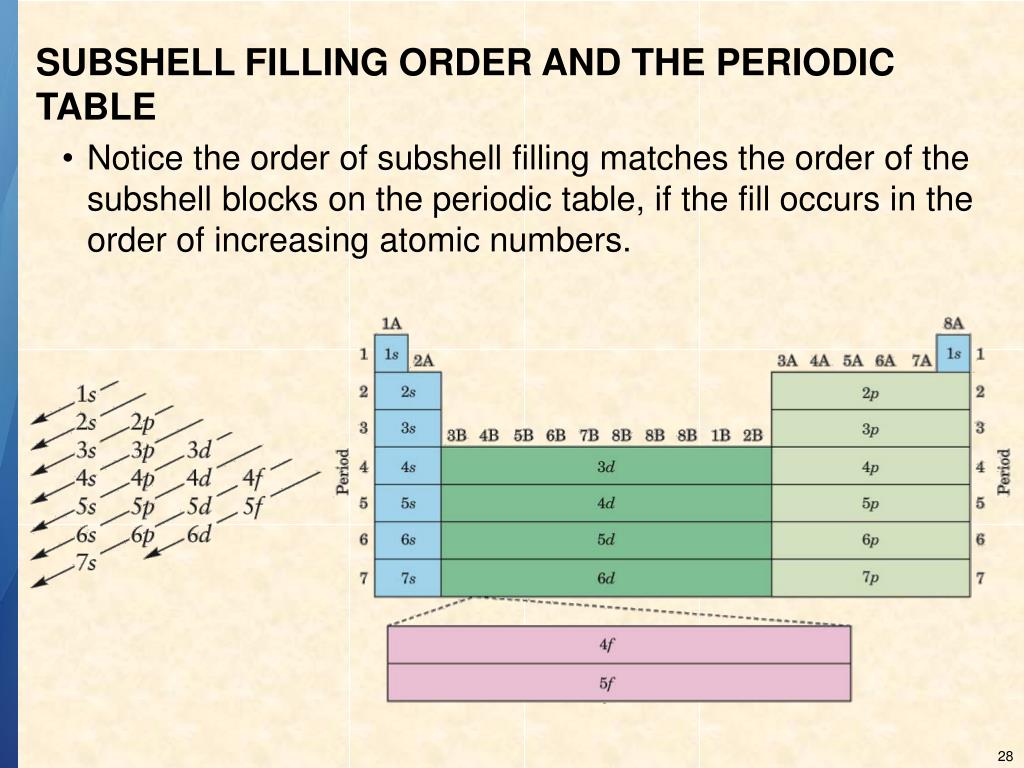
Subshell Filling Rules . when writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons. The placement of electrons in atoms is complex and there are several levels of organisation. larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. These four orbitals can contain eight electrons. the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). For example, after filling the 3p block up to.
from www.slideserve.com
larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. when writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons. The placement of electrons in atoms is complex and there are several levels of organisation. For example, after filling the 3p block up to. These four orbitals can contain eight electrons. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of.
PPT Chapter 3 Electron Structure and the Periodic Law PowerPoint
Subshell Filling Rules larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of. when writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons. For example, after filling the 3p block up to. the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). These four orbitals can contain eight electrons. larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. The placement of electrons in atoms is complex and there are several levels of organisation.
From www.youtube.com
SSLC // filling of electrons in subshell // Table 1.6 YouTube Subshell Filling Rules The placement of electrons in atoms is complex and there are several levels of organisation. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. hund's rule states that orbitals of equal. Subshell Filling Rules.
From www.slideserve.com
PPT Unit 33 Electron configuration PowerPoint Presentation, free Subshell Filling Rules larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of. the filling order simply begins at hydrogen and includes each subshell. Subshell Filling Rules.
From www.youtube.com
In which `5f` subshell is halffilled? YouTube Subshell Filling Rules hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of. These four orbitals can contain eight electrons. For example, after filling the 3p block up to. the filling order simply begins at hydrogen and includes each subshell as you proceed in. Subshell Filling Rules.
From mavink.com
Orbital Subshell Chart Subshell Filling Rules larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. when writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons. These four orbitals can contain eight electrons. For example, after filling the. Subshell Filling Rules.
From www.slideserve.com
PPT Ch. 8 Periodic Properties of the Elements PowerPoint Presentation Subshell Filling Rules hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of. larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. The placement of electrons in atoms is complex and there are several. Subshell Filling Rules.
From slidetodoc.com
Electron Configurations Using Subshell Notation and Orbital Diagrams Subshell Filling Rules The placement of electrons in atoms is complex and there are several levels of organisation. larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. For example, after filling the 3p. Subshell Filling Rules.
From www.youtube.com
Science Tutorial Writing the Electron Configuration Subshell Notation Subshell Filling Rules The placement of electrons in atoms is complex and there are several levels of organisation. These four orbitals can contain eight electrons. For example, after filling the 3p block up to. larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. the filling order simply begins at hydrogen and. Subshell Filling Rules.
From www.peoi.org
Chapter 8 Section C Organization of Electrons in Atoms Subshell Filling Rules The placement of electrons in atoms is complex and there are several levels of organisation. larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. when writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the. Subshell Filling Rules.
From physicsteacher.in
Electron shells for the first 20 elements & shells filling rules Subshell Filling Rules hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of. The placement of electrons in atoms is complex and there are several levels of organisation. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled. Subshell Filling Rules.
From www.peoi.org
Chapter 8 Section D Electronic Structure and the Periodic Table Subshell Filling Rules hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). the filling order simply begins at hydrogen and includes each subshell as you. Subshell Filling Rules.
From www.sliderbase.com
Atomic Subshell Energies Subshell Filling Rules when writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons. For example, after filling the 3p block up to. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). the filling order simply. Subshell Filling Rules.
From www.slideserve.com
PPT Subshells and Orbitals PowerPoint Presentation, free download Subshell Filling Rules hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of. when writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons. These four orbitals can. Subshell Filling Rules.
From mungfali.com
Orbital Subshell Chart Subshell Filling Rules The placement of electrons in atoms is complex and there are several levels of organisation. larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. For example, after filling the 3p block up to. when writing an electron configuration, first write the energy level (the period), then the subshell. Subshell Filling Rules.
From edu.rsc.org
How to teach electron configurations Poster RSC Education Subshell Filling Rules hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of. larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. For example, after filling the 3p block up to. the filling. Subshell Filling Rules.
From ar.inspiredpencil.com
Electron Subshell Subshell Filling Rules These four orbitals can contain eight electrons. The placement of electrons in atoms is complex and there are several levels of organisation. the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). . Subshell Filling Rules.
From www.youtube.com
Trick to learn the order of energy of Subshells Aufbau Principle n+l Subshell Filling Rules larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. These four orbitals can contain eight electrons. hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of. (a) when n = 2,. Subshell Filling Rules.
From www.toppr.com
The subshell which are filled just before and just after the filling of Subshell Filling Rules (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. For example, after filling the 3p block up to. the filling order simply begins at hydrogen and includes each subshell as. Subshell Filling Rules.
From www.numerade.com
SOLVED Indicate the position the periodic table where each of the Subshell Filling Rules the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. when writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons. The placement of electrons in atoms is complex and there are several levels. Subshell Filling Rules.
From www.slideserve.com
PPT Electron Configurations PowerPoint Presentation, free download Subshell Filling Rules The placement of electrons in atoms is complex and there are several levels of organisation. the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). These four orbitals can contain eight electrons. . Subshell Filling Rules.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Subshell Filling Rules the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. These four orbitals can contain eight electrons. The placement of electrons in atoms is complex and there are several levels of organisation. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). For. Subshell Filling Rules.
From www.teachmint.com
Subshell Filling (Aufbau Principle) 11th Chemistry Class Recording Subshell Filling Rules These four orbitals can contain eight electrons. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). For example, after filling the 3p block up to. the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. hund's rule states that orbitals of. Subshell Filling Rules.
From www.slideserve.com
PPT Chapter 8 Periodic Properties of the Elements PowerPoint Subshell Filling Rules (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). These four orbitals can contain eight electrons. the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. The placement of electrons in atoms is complex and there are several levels of organisation. For. Subshell Filling Rules.
From chemistryguru.com.sg
3 Rules for Electronic Configuration using Quantum Model Subshell Filling Rules the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. The placement of electrons in atoms is complex and there are several levels of organisation. These four orbitals can contain eight electrons. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). . Subshell Filling Rules.
From hollyimg07.blogspot.com
Electron Subshell Flow Diagram Periodic Table Blocks Of Elements Subshell Filling Rules (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of. when writing an electron configuration, first write the energy level (the period), then. Subshell Filling Rules.
From www.bartleby.com
Answered Show the orbitalfilling diagram for N… bartleby Subshell Filling Rules when writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons. These four orbitals can contain eight electrons. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). For example, after filling the 3p block. Subshell Filling Rules.
From www.slideserve.com
PPT Radiation and Energy PowerPoint Presentation Subshell Filling Rules (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. The placement of. Subshell Filling Rules.
From www.breakingatom.com
Subshells Definition Subshell Filling Rules For example, after filling the 3p block up to. The placement of electrons in atoms is complex and there are several levels of organisation. the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled. Subshell Filling Rules.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry Subshell Filling Rules larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a. Subshell Filling Rules.
From www.chegg.com
Solved Show the orbitalfilling diagram for N (nitrogen). Subshell Filling Rules The placement of electrons in atoms is complex and there are several levels of organisation. the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. For example, after filling the 3p block up to. when writing an electron configuration, first write the energy level (the period), then the subshell to. Subshell Filling Rules.
From chem.libretexts.org
7.2 Atomic Subshell Energies and Electron Assignments Chemistry Subshell Filling Rules when writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons. hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of. (a) when n =. Subshell Filling Rules.
From www.slideserve.com
PPT Chapter 3 Electron Structure and the Periodic Law PowerPoint Subshell Filling Rules These four orbitals can contain eight electrons. hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of. (a) when n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). The placement of electrons in atoms is. Subshell Filling Rules.
From www.sliderbase.com
Atomic Subshell Energies Subshell Filling Rules larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. These four orbitals can contain eight electrons. when writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons. (a) when n = 2,. Subshell Filling Rules.
From slideplayer.com
Order in which subshells are filled with electrons ppt download Subshell Filling Rules For example, after filling the 3p block up to. the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. larger atoms with more subshells will seem to fill out of order, as the other factors influencing orbital energy. when writing an electron configuration, first write the energy level (the. Subshell Filling Rules.
From www.slideserve.com
PPT Carbon Compounds and Chemical Bonds PowerPoint Presentation, free Subshell Filling Rules For example, after filling the 3p block up to. when writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons. The placement of electrons in atoms is complex and there are several levels of organisation. These four orbitals can contain eight electrons. . Subshell Filling Rules.
From chem.libretexts.org
7.2 Atomic Subshell Energies and Electron Assignments Chemistry Subshell Filling Rules when writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons. the filling order simply begins at hydrogen and includes each subshell as you proceed in increasing z order. hund's rule states that orbitals of equal energy are each occupied by. Subshell Filling Rules.