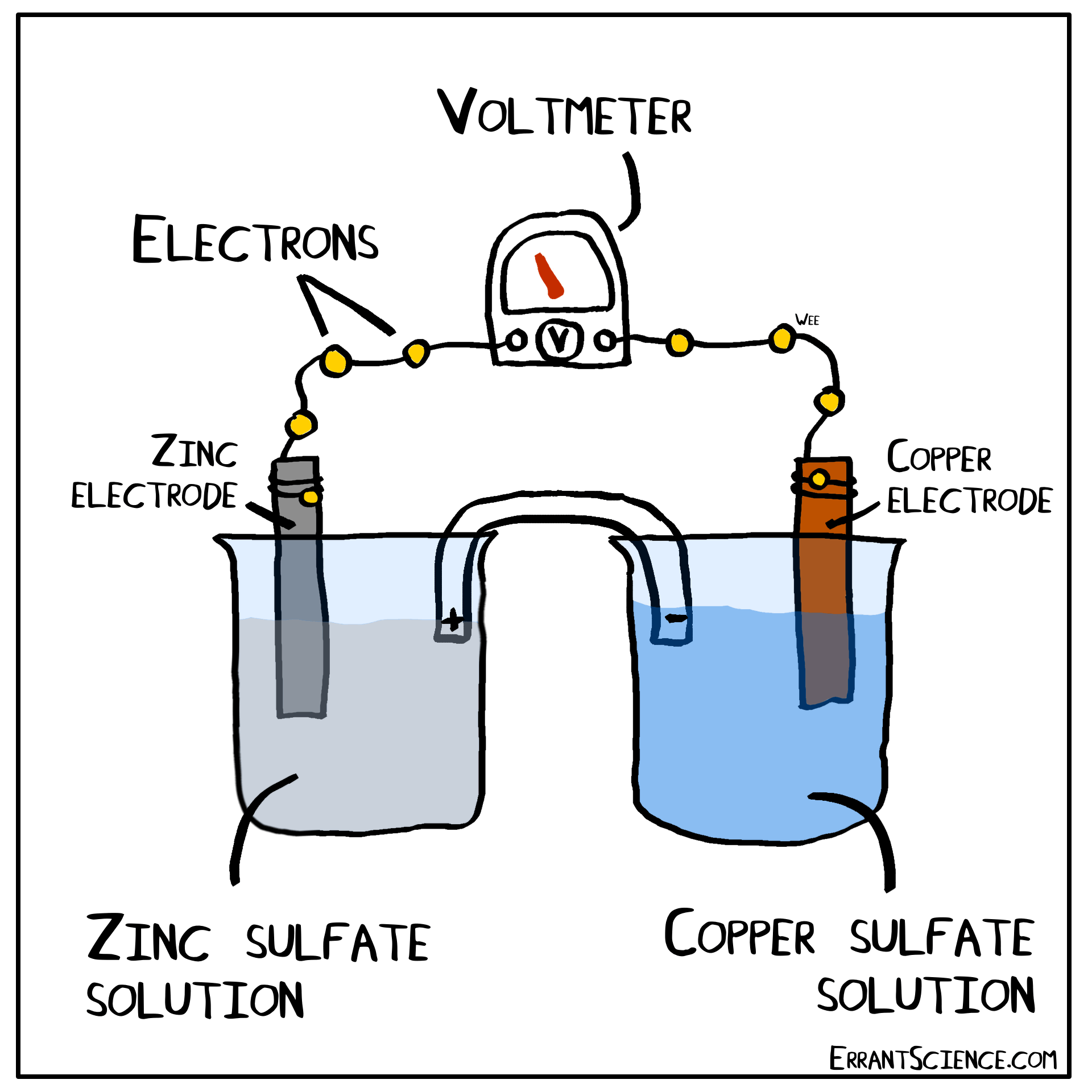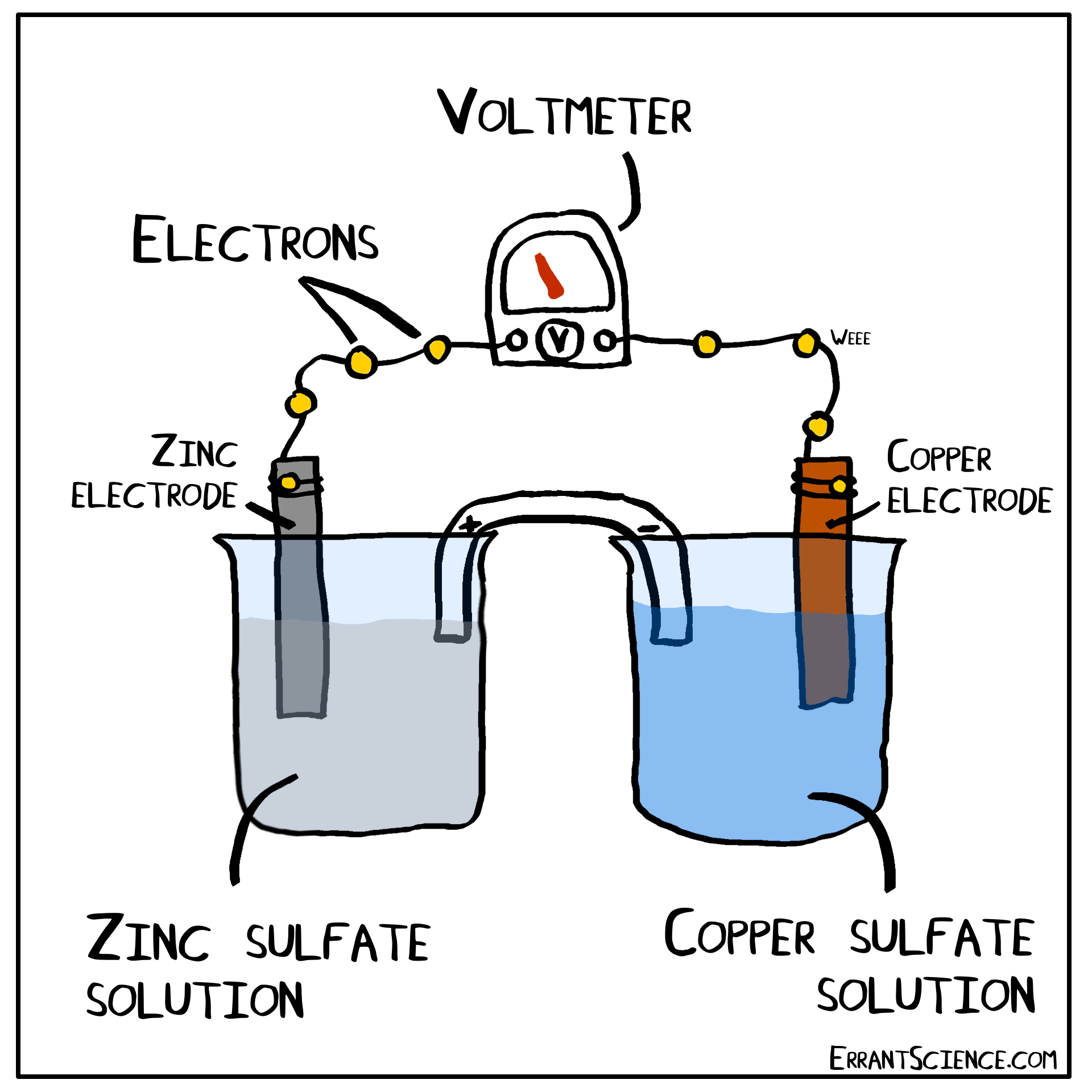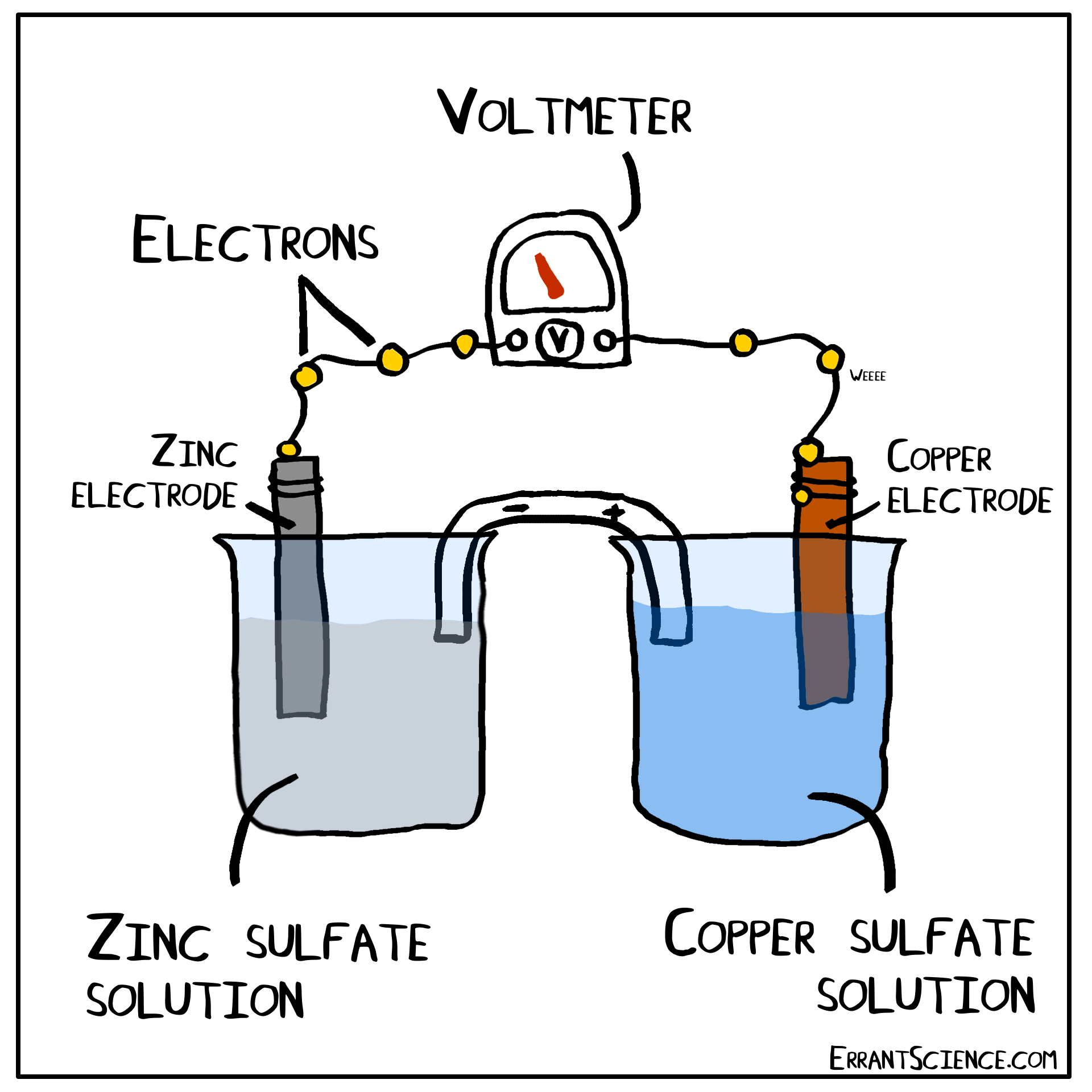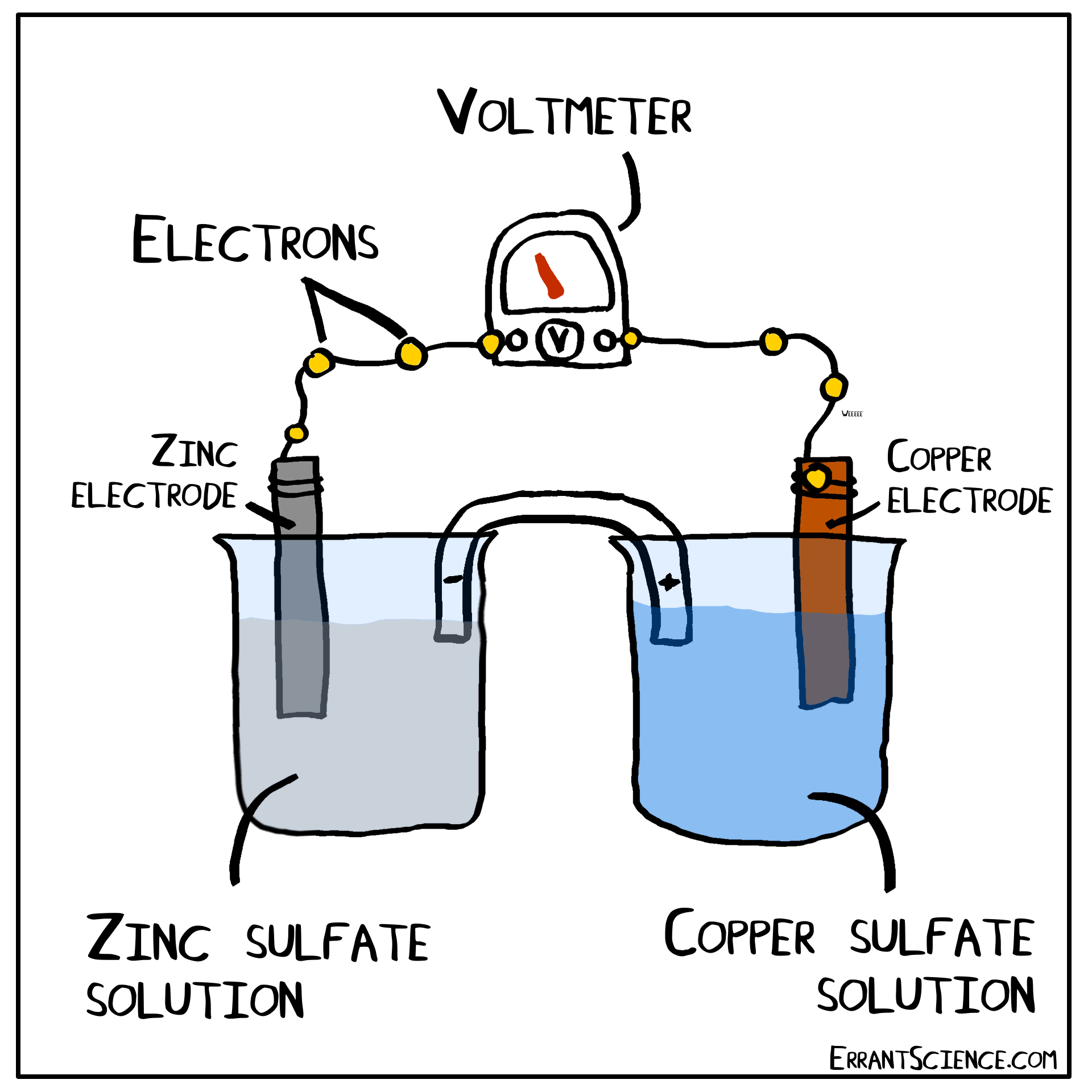
Examples Of Electrochemical Cell Reaction . An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. There are two types of electrochemical cells: Electrochemical reactions are those in which electric currents are either generated or input. Galvanic cells and electrolytic cells. These responses can be broadly divided into two categories: When electrons transfer from one element to another during specific types of reactions, electricity can result (such as redox reactions). Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. The operating principle of the voltaic cell is a simultaneous oxidation and reduction reaction, called a redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to.
from generic.wordpress.soton.ac.uk
The operating principle of the voltaic cell is a simultaneous oxidation and reduction reaction, called a redox reaction. Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. Galvanic cells and electrolytic cells. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Electrochemical reactions are those in which electric currents are either generated or input. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. There are two types of electrochemical cells: An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. When electrons transfer from one element to another during specific types of reactions, electricity can result (such as redox reactions). These responses can be broadly divided into two categories:
Electrochemistry explanations, videos and everyday life examples
Examples Of Electrochemical Cell Reaction An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Electrochemical reactions are those in which electric currents are either generated or input. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Galvanic cells and electrolytic cells. When electrons transfer from one element to another during specific types of reactions, electricity can result (such as redox reactions). The operating principle of the voltaic cell is a simultaneous oxidation and reduction reaction, called a redox reaction. These responses can be broadly divided into two categories: Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. There are two types of electrochemical cells:
From saylordotorg.github.io
Electrochemistry Examples Of Electrochemical Cell Reaction When electrons transfer from one element to another during specific types of reactions, electricity can result (such as redox reactions). These responses can be broadly divided into two categories: Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. There are two types of electrochemical cells: Electrochemical reactions are those in which. Examples Of Electrochemical Cell Reaction.
From www.slideserve.com
PPT Electrochemical Cells Voltage (Electric potential) PowerPoint Examples Of Electrochemical Cell Reaction The operating principle of the voltaic cell is a simultaneous oxidation and reduction reaction, called a redox reaction. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Electrochemical reactions are those in which electric currents are either generated or input. When electrons transfer. Examples Of Electrochemical Cell Reaction.
From mmerevise.co.uk
Electrochemical Cells MME Examples Of Electrochemical Cell Reaction The operating principle of the voltaic cell is a simultaneous oxidation and reduction reaction, called a redox reaction. There are two types of electrochemical cells: Galvanic cells and electrolytic cells. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Electrochemical cells consist of. Examples Of Electrochemical Cell Reaction.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in Examples Of Electrochemical Cell Reaction When electrons transfer from one element to another during specific types of reactions, electricity can result (such as redox reactions). Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Electrochemical reactions are those in which electric currents are either generated or input. There. Examples Of Electrochemical Cell Reaction.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and Examples Of Electrochemical Cell Reaction There are two types of electrochemical cells: An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. These responses can be broadly divided into two categories: Galvanic cells and electrolytic cells. Electrochemical reactions are those in which electric currents are either generated or input. The operating principle of the voltaic cell is a simultaneous oxidation. Examples Of Electrochemical Cell Reaction.
From generic.wordpress.soton.ac.uk
Electrochemistry explanations, videos and everyday life examples Examples Of Electrochemical Cell Reaction Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Electrochemical reactions are those in which electric currents are either generated or input. When electrons transfer from one element to another during specific types of reactions, electricity can result (such as redox reactions). Electrochemical. Examples Of Electrochemical Cell Reaction.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Examples Of Electrochemical Cell Reaction These responses can be broadly divided into two categories: Electrochemical reactions are those in which electric currents are either generated or input. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. The operating principle of the voltaic cell is a. Examples Of Electrochemical Cell Reaction.
From mavink.com
Diagram Of Electrochemical Cell Examples Of Electrochemical Cell Reaction Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. Electrochemical reactions are those in which electric currents are either generated or input. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Galvanic cells and. Examples Of Electrochemical Cell Reaction.
From www.slideserve.com
PPT ELECTROCHEMISTRY PowerPoint Presentation, free download ID5580086 Examples Of Electrochemical Cell Reaction Galvanic cells and electrolytic cells. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. The operating principle of the voltaic cell is a simultaneous oxidation and reduction reaction, called a redox reaction. When electrons transfer from one element to another during specific types. Examples Of Electrochemical Cell Reaction.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Examples Of Electrochemical Cell Reaction The operating principle of the voltaic cell is a simultaneous oxidation and reduction reaction, called a redox reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. These responses can be broadly divided into two categories: Galvanic cells and electrolytic. Examples Of Electrochemical Cell Reaction.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii Examples Of Electrochemical Cell Reaction Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. These responses can be broadly divided into two categories: An electrochemical cell splits. Examples Of Electrochemical Cell Reaction.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Examples Of Electrochemical Cell Reaction Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Electrochemical reactions are those in which electric currents are either generated or input. Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. An apparatus that. Examples Of Electrochemical Cell Reaction.
From www.youtube.com
GCSE CHEMISTRY ELECTRO CHEMISTRY LESSON 10 simple voltaic cell Examples Of Electrochemical Cell Reaction The operating principle of the voltaic cell is a simultaneous oxidation and reduction reaction, called a redox reaction. Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons. Examples Of Electrochemical Cell Reaction.
From www.slideserve.com
PPT Introduction to electrochemical systems PowerPoint Presentation Examples Of Electrochemical Cell Reaction Galvanic cells and electrolytic cells. Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. There are two types of electrochemical cells: Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. An electrochemical cell splits. Examples Of Electrochemical Cell Reaction.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Examples Of Electrochemical Cell Reaction When electrons transfer from one element to another during specific types of reactions, electricity can result (such as redox reactions). Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. Galvanic cells and electrolytic cells. There are two types of electrochemical cells: Electrochemical reactions are those in which electric currents are either. Examples Of Electrochemical Cell Reaction.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Examples Of Electrochemical Cell Reaction Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. There are two types of electrochemical cells: Electrochemical reactions are those in which electric currents are either generated or input. Galvanic cells and electrolytic cells. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. Examples Of Electrochemical Cell Reaction.
From wisc.pb.unizin.org
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109 Examples Of Electrochemical Cell Reaction These responses can be broadly divided into two categories: Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Galvanic cells and electrolytic cells. There are two types of. Examples Of Electrochemical Cell Reaction.
From www.researchgate.net
Example of an electrochemical cell consisting of two electrolytic Examples Of Electrochemical Cell Reaction The operating principle of the voltaic cell is a simultaneous oxidation and reduction reaction, called a redox reaction. Galvanic cells and electrolytic cells. Electrochemical reactions are those in which electric currents are either generated or input. These responses can be broadly divided into two categories: Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox. Examples Of Electrochemical Cell Reaction.
From saylordotorg.github.io
Describing Electrochemical Cells Examples Of Electrochemical Cell Reaction These responses can be broadly divided into two categories: Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. Galvanic cells and electrolytic cells. When electrons. Examples Of Electrochemical Cell Reaction.
From pubs.rsc.org
Making electrochemistry easily accessible to the synthetic chemist Examples Of Electrochemical Cell Reaction When electrons transfer from one element to another during specific types of reactions, electricity can result (such as redox reactions). Galvanic cells and electrolytic cells. Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. There are. Examples Of Electrochemical Cell Reaction.
From stoplearn.com
Electrochemical Cells 2023 Examples Of Electrochemical Cell Reaction There are two types of electrochemical cells: Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. Galvanic cells and electrolytic cells. Electrochemical reactions are those in which electric currents are either generated or input. An apparatus that is used to generate electricity from. Examples Of Electrochemical Cell Reaction.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson Examples Of Electrochemical Cell Reaction Galvanic cells and electrolytic cells. Electrochemical reactions are those in which electric currents are either generated or input. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. There are two types of electrochemical cells: An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive. Examples Of Electrochemical Cell Reaction.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Examples Of Electrochemical Cell Reaction When electrons transfer from one element to another during specific types of reactions, electricity can result (such as redox reactions). An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. These responses can be broadly divided into two categories: Electrochemical reaction,. Examples Of Electrochemical Cell Reaction.
From studylib.net
Electrochemical cells Examples Of Electrochemical Cell Reaction Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. There are two types of electrochemical cells: The operating principle of the voltaic cell is a simultaneous oxidation and reduction reaction, called a redox reaction. An electrochemical cell splits the oxidant and reductant in. Examples Of Electrochemical Cell Reaction.
From knowworldnow.com
Redox Reactions & Electrochemical Cells in OCR A Level Chemistry Know Examples Of Electrochemical Cell Reaction An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. These responses can be broadly divided into two categories: An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. There are two types of electrochemical cells:. Examples Of Electrochemical Cell Reaction.
From www.pinterest.com
Electrochemical cell Electrochemistry, Chemistry classroom Examples Of Electrochemical Cell Reaction An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. Galvanic cells and electrolytic cells. When electrons transfer from one element to another during specific types of reactions, electricity can result (such as redox reactions). An apparatus. Examples Of Electrochemical Cell Reaction.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID541675 Examples Of Electrochemical Cell Reaction An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. When electrons transfer from one element to another during specific types of reactions, electricity can result (such as redox reactions). The operating principle of the voltaic cell is a simultaneous oxidation and reduction reaction, called a redox reaction. An apparatus that is used to generate. Examples Of Electrochemical Cell Reaction.
From in.pinterest.com
What is Electrochemical Cell Notation Line notation Cell Diagram Examples Of Electrochemical Cell Reaction An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. There are two types of electrochemical cells: Galvanic cells and electrolytic cells. These responses can be broadly divided into two categories: Electrochemical reaction, any process either caused or accompanied by the. Examples Of Electrochemical Cell Reaction.
From www.youtube.com
electrochemical cell electrochemistry class 12 chemistry subject notes Examples Of Electrochemical Cell Reaction When electrons transfer from one element to another during specific types of reactions, electricity can result (such as redox reactions). Electrochemical reactions are those in which electric currents are either generated or input. There are two types of electrochemical cells: These responses can be broadly divided into two categories: An electrochemical cell splits the oxidant and reductant in a manner. Examples Of Electrochemical Cell Reaction.
From www.youtube.com
Electrochemistry 04 Writing Electrochemical Cell Notation YouTube Examples Of Electrochemical Cell Reaction There are two types of electrochemical cells: When electrons transfer from one element to another during specific types of reactions, electricity can result (such as redox reactions). Electrochemical reactions are those in which electric currents are either generated or input. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases. Examples Of Electrochemical Cell Reaction.
From www.collegesearch.in
Electrochemical Cell Definitions, Examples, Electrochemistry Examples Of Electrochemical Cell Reaction Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. When electrons transfer from one element to another during specific types of reactions, electricity can result (such as redox. Examples Of Electrochemical Cell Reaction.
From www.thoughtco.com
Electrochemical Cell Definition Examples Of Electrochemical Cell Reaction Electrochemical reactions are those in which electric currents are either generated or input. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer of electrons between two. There are two types of electrochemical cells: When electrons transfer from one element to another during specific types of reactions, electricity. Examples Of Electrochemical Cell Reaction.
From saylordotorg.github.io
Electrochemistry Examples Of Electrochemical Cell Reaction An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. Galvanic cells and electrolytic cells. Electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. Examples Of Electrochemical Cell Reaction.
From www.alamy.com
Simple electrochemical or galvanic cell. The Daniell cell Stock Photo Examples Of Electrochemical Cell Reaction An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. Electrochemical reactions are those in which electric currents are either generated or input. Galvanic cells and electrolytic cells. The operating principle of the voltaic cell is a simultaneous oxidation and reduction. Examples Of Electrochemical Cell Reaction.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Examples Of Electrochemical Cell Reaction Electrochemical cells consist of two electrodes (conducting materials that serve as the site for redox reactions), a solution. Electrochemical reactions are those in which electric currents are either generated or input. Galvanic cells and electrolytic cells. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Examples Of Electrochemical Cell Reaction.