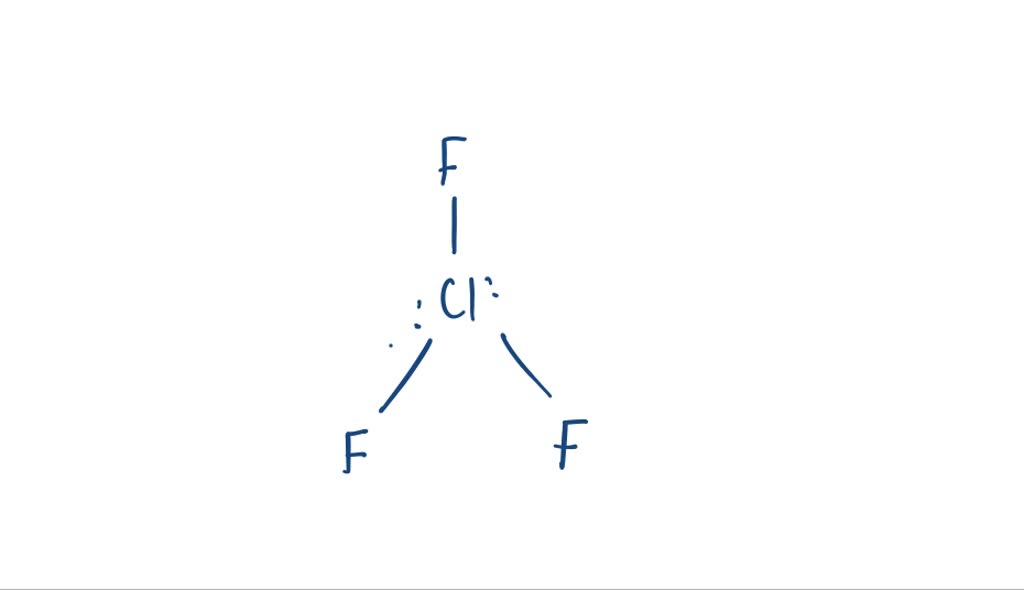
Chlorine Trifluoride Reaction Equation . Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Chlorine + fluorine = chlorine trifluoride. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in all. Chlorine trifluoride = dichlorine + difluorine. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three moles. Chlorine and fluorine are nasty enough by themselves, but at least in.
from www.numerade.com
Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride = dichlorine + difluorine. Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three moles. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in all. Chlorine + fluorine = chlorine trifluoride.
SOLVEDChlorine trifluoride, CIF3, is one of the most reactive
Chlorine Trifluoride Reaction Equation Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three moles. Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three moles. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in all. Chlorine + fluorine = chlorine trifluoride. Chlorine trifluoride = dichlorine + difluorine. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride.
From www.youtube.com
ClF3 (Chlorine trifluoride) Molecular Geometry, Bond Angles,& Electron Chlorine Trifluoride Reaction Equation Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine trifluoride = dichlorine + difluorine. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high. Chlorine Trifluoride Reaction Equation.
From ar.inspiredpencil.com
Lewis Structure Of Fluorine Ion Chlorine Trifluoride Reaction Equation Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in. Chlorine Trifluoride Reaction Equation.
From www.youtube.com
How to Balance F2 + Cl2 = ClF3 (Fluorine gas + Chlorine gas) YouTube Chlorine Trifluoride Reaction Equation Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Chlorine trifluoride = dichlorine + difluorine. Chlorine and fluorine are nasty enough by themselves, but at least in. Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three. Chlorine Trifluoride Reaction Equation.
From www.slideserve.com
PPT VSEPR. PowerPoint Presentation ID214953 Chlorine Trifluoride Reaction Equation Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine + fluorine = chlorine trifluoride. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Chlorine trifluoride is an extremely reactive, corrosive and irritating. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVED Chlorine gas reacts with fluorine gas to form chlorine Chlorine Trifluoride Reaction Equation Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three moles. Chlorine trifluoride = dichlorine + difluorine. Clf3 = cl2 + f2 is a decomposition reaction where two. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVED The reaction of hydrazine (N2H4) and chlorine trifluoride (ClF3 Chlorine Trifluoride Reaction Equation Chlorine trifluoride = dichlorine + difluorine. Chlorine + fluorine = chlorine trifluoride. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in all. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine trifluoride (which i will subsequently refer. Chlorine Trifluoride Reaction Equation.
From interestingengineering.com
Chlorine Trifluoride The Compound That Can Even Set Fire to Glass Chlorine Trifluoride Reaction Equation Chlorine trifluoride = dichlorine + difluorine. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high. Chlorine Trifluoride Reaction Equation.
From www.coursehero.com
[Solved] The reaction of hydrazine, N2H4, with chlorine trifluoride Chlorine Trifluoride Reaction Equation Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in all. Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three moles. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVED Ammonia gas reacts with chlorine trifluoride gas to produce Chlorine Trifluoride Reaction Equation Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride = dichlorine + difluorine. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in all. Cl + f = clf3 is a synthesis. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
Chlorine and fluorine react to form gaseous chlorine trifluoride Chlorine Trifluoride Reaction Equation Chlorine trifluoride = dichlorine + difluorine. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three moles. Chlorine. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVED Chlorine gas reacts with fluorine gas t0 form chlorine Chlorine Trifluoride Reaction Equation Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in all. Chlorine trifluoride. Chlorine Trifluoride Reaction Equation.
From www.youtube.com
ClF3 Hybridization (Chlorine Trifluoride) YouTube Chlorine Trifluoride Reaction Equation Chlorine trifluoride = dichlorine + difluorine. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in all. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most. Chlorine Trifluoride Reaction Equation.
From felica8sy-images.blogspot.com
Fcl3 Lewis Structure / Vsepr Clf3 Chlorine Trifluoride / Give each of Chlorine Trifluoride Reaction Equation Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in all. Clf3 = cl2 + f2 is a decomposition reaction. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVED Chlorine gas reacts with fluorine gas to form chlorine Chlorine Trifluoride Reaction Equation Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three moles. Chlorine trifluoride = dichlorine + difluorine. Chlorine + fluorine = chlorine trifluoride. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVEDChlorine trifluoride, ClF3, is a valuable reagent because it can Chlorine Trifluoride Reaction Equation Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in all. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVEDChlorine trifluoride, CIF3, is one of the most reactive Chlorine Trifluoride Reaction Equation Chlorine + fluorine = chlorine trifluoride. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Cl + f = clf3 is a synthesis reaction. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons Chlorine Trifluoride Reaction Equation Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three moles. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVEDThe reaction of hydrazine, N2 H4, with chlorine trifluoride Chlorine Trifluoride Reaction Equation Chlorine trifluoride = dichlorine + difluorine. Chlorine and fluorine are nasty enough by themselves, but at least in. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in all. Chlorine. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVED Gaseous chlorine trifluoride is a compound used in the Chlorine Trifluoride Reaction Equation Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Chlorine + fluorine = chlorine trifluoride. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in all. Chlorine trifluoride (which. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVED 1) Chlorine gas reacts with fluorine gas to produce chlorine Chlorine Trifluoride Reaction Equation Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three moles. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVED The production of chlorine trifluoride is given by the equation Chlorine Trifluoride Reaction Equation Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in all. Chlorine trifluoride = dichlorine + difluorine. Cl + f. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVED Chlorine gas reacts with fluorine gas to form chlorine Chlorine Trifluoride Reaction Equation Chlorine trifluoride = dichlorine + difluorine. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high. Chlorine Trifluoride Reaction Equation.
From de-academic.com
Chlor(III)fluorid Chlorine Trifluoride Reaction Equation Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three moles. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine and fluorine are nasty enough by. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVED Consider the reaction between chlorine trifluoride gas, CIFs(g Chlorine Trifluoride Reaction Equation Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Chlorine trifluoride = dichlorine + difluorine. Chlorine and fluorine are nasty enough by themselves, but at least in. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Cl +. Chlorine Trifluoride Reaction Equation.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Chlorine Trifluoride Reaction Equation Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride = dichlorine + difluorine. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons Chlorine Trifluoride Reaction Equation Chlorine and fluorine are nasty enough by themselves, but at least in. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Chlorine trifluoride (which i will subsequently refer to. Chlorine Trifluoride Reaction Equation.
From www.chegg.com
Solved 1. 4/6 points Chlorine trifluoride is a highly Chlorine Trifluoride Reaction Equation Chlorine trifluoride = dichlorine + difluorine. Chlorine + fluorine = chlorine trifluoride. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has. Chlorine Trifluoride Reaction Equation.
From www.studocu.com
Chlorine Trifluoride M.O. Diagram CHEM12A Studocu Chlorine Trifluoride Reaction Equation Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Chlorine trifluoride = dichlorine + difluorine. Chlorine + fluorine = chlorine trifluoride. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine trifluoride (which i will subsequently refer to. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
⏩SOLVEDChlorine gas reacts with fluorine gas to form chlorine… Numerade Chlorine Trifluoride Reaction Equation Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in all. Chlorine trifluoride. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVED Chlorine gas reacts with fluorine gas to form chlorine Chlorine Trifluoride Reaction Equation Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three moles. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride = dichlorine + difluorine. Chlorine + fluorine = chlorine trifluoride. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVED Question 9 1 pts Chlorine gas reacts with fluorine gas to form Chlorine Trifluoride Reaction Equation Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three moles. Clf3 = cl2 + f2. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVED Chlorine gas reacts with fluorine gas to form chlorine Chlorine Trifluoride Reaction Equation Chlorine + fluorine = chlorine trifluoride. Chlorine trifluoride = dichlorine + difluorine. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Cl + f = clf3 is a synthesis. Chlorine Trifluoride Reaction Equation.
From www.chegg.com
Solved 04 0/3 points Chlorine trifluoride is a highly Chlorine Trifluoride Reaction Equation Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine + fluorine = chlorine trifluoride. Clf3 = cl2 + f2 is a decomposition reaction where two moles of chlorine trifluoride. Chlorine trifluoride = dichlorine + difluorine. Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three moles. Chlorine trifluoride (which. Chlorine Trifluoride Reaction Equation.
From www.chegg.com
Solved 5. Molecule CIF, (chlorine trifluoride is an Chlorine Trifluoride Reaction Equation Chlorine + fluorine = chlorine trifluoride. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride = dichlorine + difluorine. Chlorine trifluoride (cf 3, bp = 11.75 °c) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and. Chlorine trifluoride is. Chlorine Trifluoride Reaction Equation.
From www.numerade.com
SOLVEDReaction of gaseous fluorine wilh gaseous chlorire fluoride orly Chlorine Trifluoride Reaction Equation Chlorine trifluoride is an extremely reactive, corrosive and irritating gas which experimentally has been observed to cause a severe reaction in the lungs and in all. Chlorine trifluoride = dichlorine + difluorine. Chlorine + fluorine = chlorine trifluoride. Cl + f = clf3 is a synthesis reaction where one mole of chlorine [cl] and three moles. Clf3 = cl2 +. Chlorine Trifluoride Reaction Equation.